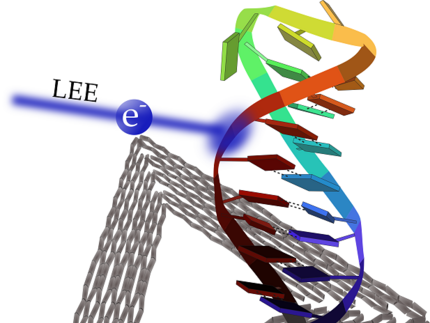
DNA radiation damage probed by DNA nanotechnology
Overview
Ionizing radiation is used in cancer radiation therapy to effectively damage the DNA of tumors. The main damage is due to generation of highly reactive secondary species such as low-energy electrons (LEEs). The accurate quantification of DNA radiation damage of well-defined DNA target sequences in terms of absolute cross sections for LEE-induced DNA strand breaks is possible by the DNA origami technique; however, to date, it is possible only for DNA single strands. In the present work DNA double strand breaks in the DNA sequence 5′-d(CAC)4/5′- d(GTG)4 are compared with DNA single strand breaks in the oligonucleotides 5′-d(CAC)4 and 5′-d(GTG)4 upon irradiation with LEEs in the energy range from 5 to 20 eV. A maximum of strand break cross section was found around 7 and 10 eV independent of the DNA sequence, indicating that dissociative electron attachment is the underlying mechanism of strand breakage and confirming previous studies using plasmid DNA.
Selected publications
Length and Energy Dependence of Low-Energy Electron-Induced Strand Breaks in Poly(A) DNA K. Ebel & I. Bald Int. J. Mol. Sci. 2020, 21, 111.
Vacuum-UV and Low-Energy Electron Induced DNA Strand Breaks – Influence of the DNA Sequence and Substrate, S. Vogel, K. Ebel, R. Schürmann, C. Heck, T. Meiling, A. Milosavljević, A. Giuliani, I. Bald, ChemPhysChem 2019, 20, 823-830. Cover: 10.1002/cphc.201900204.
Vacuum-UV induced DNA strand breaks - influence of the radiosensitizers 5-bromouracil and 8-bromoadenine, S. Vogel, K. Ebel, C. Heck, R. Schürmann, A. Milosavljević, A. Giuliani, I. Bald, Phys. Chem. Chem. Phys., 2019, 21, 1972.
The Physico-Chemical Basis of DNA Radiosensitization: Implications for Cancer Radiation Therapy R. Schürmann, S. Vogel, K. Ebel & I. Bald Chem. Eur. J. 2018, 24, 10271-10279.
Low-Energy Electron-Induced Strand Breaks in Telomere-Derived DNA Sequences - Influence of DNA Sequence and Topology, J. Rackwitz, I. Bald, Chem. Eur. J. 2018, 24, 4680. See also a feature article in Chemistry Views.
Resonant formation of strand breaks in sensitized oligonucleotides induced by low-energy electrons (0.5 - 9.0 eV); R. Schürmann, T. Tsering, K. Tanzer, S. Denifl, S. V. K. Kumar, I. Bald, Angew. Chem. Int. Ed. 2017, 56, 10952.
A novel setup for the determination of absolute cross sections for low-energy electron induced strand breaks in oligonucleotides – The effect of the radiosensitizer 5-fluorouracil; J. Rackwitz, M. Lj. Rankovic, A. R. Milosavljevic, I. Bald, Eur. Phys. J. D 2017, 71, 32.
Sensitizing DNA Towards Low-Energy Electrons with 2-Fluoroadenine; J. Rackwitz, J. Kopyra, I. Dabkowska, K. Ebel, M. Lj. Rankovic, A. R. Milosavljevic, I. Bald, Angew. Chem. Int. Ed. 2016, 55, 10248.
Using DNA Origami Nanostructures To Determine Absolute Cross Sections for UV Photon-Induced DNA Strand Breakage; S. Vogel, J. Rackwitz, R. Schürman, J. Prinz, A. R. Milosavljević, M. Réfrégiers, A. Giuliani, I. Bald, J. Phys. Chem. Lett. 2015, 6, 4589.
PhD theses completed on this topic
Dr. Kenny Ebel: Quantification of low-energy electron induced single and double strand breaks in well-defined DNA sequences using DNA origami nanostructures
Dr. Stefanie Vogel: Sequence dependency of photon and electron induced DNA strand breaks
Dr. Jenny Rackwitz: A novel approach to study low-energy electron-induced damage to DNA oligonucleotides
Dr. Robin Schürmann: Interaction of the potential DNA-radiosensitizer 8-bromoadenine with free and plasmonically generated electrons