Hybrid Nanostructures
Welcome to the pages of the Hybrid Nanostructures group. In our research we combine different methods from DNA nanotechnology, optical spectroscopy and scanning probe microscopy in order to study physico-chemical processes in nanoscale materials and at the single-molecule level. Apart from methods development we investigate specific questions such as the mechanisms of plasmon-induced chemical reactions, the nucleotide sequence dependence of DNA radiation damage and the mode of action of radiosensitizers that are applied in tumor radiation therapy.
We are members of the innovation center innoFSPEC and the research initiative "Elementary Processes of Light-Driven Reactions at Nanoscale Metals*.
Our recent work:
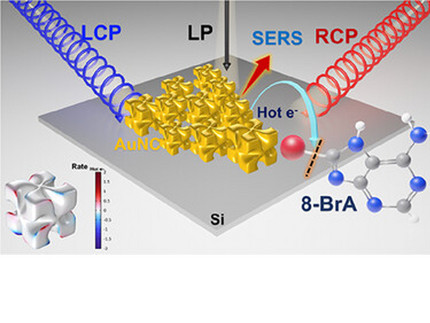
“Chiral gold nanocubes (AuNCs) enable polarization-dependent plasmonic catalysis with exceptional photochemical circular dichroism. Circularly polarized light drives hot-electron-induced reactions with asymmetric reaction rates, monitored via operando SERS, with photochemical g-factors exceeding optical limits. Multilayer assemblies reveal reversible and tunable chiroptical responses. This work offers a strategy to amplify chiral-selective photochemistry via nanostructure design and light control”.
Amplification of Photochemical Chiroptical Activity of Chiral Gold Nanocubes; S. K. Gahlaut, O. Avalos-Ovando, R. M. Kim, R. Hussein, S. Juergensen, S. Reich, A. O. Govorov, K. T. Nam, I. Bald; Small 2025, 21, no. 36, 2505093