The role of Mechanobiology in Vascular Anomalies
The etiopathogeny of CCMs remains largely unclear. We still lack a good understanding of the molecular and physiological triggers that cause acute phases of CCM lesion growth and bleeding. Other questions revolve around why this pathology is restricted to lowly-perfused venous capillary beds, and which are the best targets for interventions to suppress lesion formation and bleeding. Answering these questions will require thoroughly characterizing the molecular heterogeneity of affected endothelial cell types. One approach will be to analyze CCM-related gene programs that cause endothelial cells to change from a quiescent state to one that is proliferative and pathological. Several studies suggest that a loss of CCM proteins causes pathological changes in biomechanical signaling, which has severe consequences for cardiovascular development and physiology (Renz et al., Developmental Cell 2015; Donat et al., eLIFE 2018). That blood flow may be an important factor in the etiology of CCM pathology also stems from observations in human patients. In patients, lesions are mostly restricted to venous capillary beds, which only experience low levels of fluid shear stress.
In one study, we investigated the role of blood flow for CCM formation (Rödel et al., Circulation Research 2019). In zebrafish, a complete loss of Ccm proteins causes severe cardiac defects that result in a lack of blood flow. Without it, these mutants exhibit vascular anomalies in major blood vessels including the lateral dorsal aorta. However, when Ccm1 was rescued specifically within the heart, blood flow was restored in ccm1 mutants. This prevented overgrowth of the lateral dorsal aorta and suggests that blood flow has a vasoprotective role within strongly perfused blood vessels in CCM.
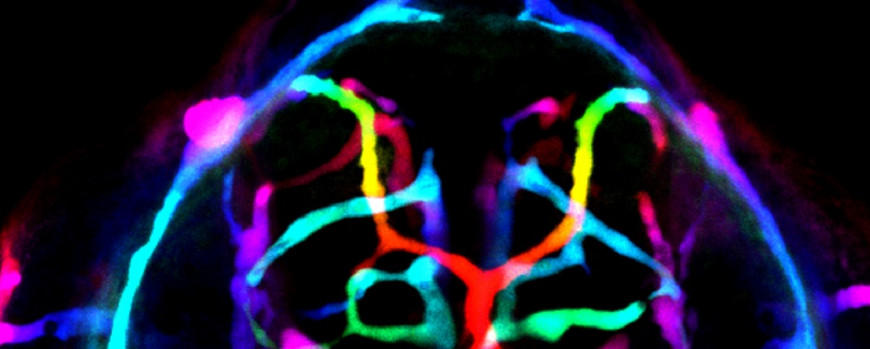
Figure 5. Microangiography reveals that the cerebrovasculature of Ccm1-deficient zebrafish embryo is leaky. Depth-color coded image shows that immediately after injection, 70 kDa Texas-Red-conjugated dextran spreads rapidly throughout the entire vasculature, outlining all vessels. On the left side, a small vascular lesion is present.