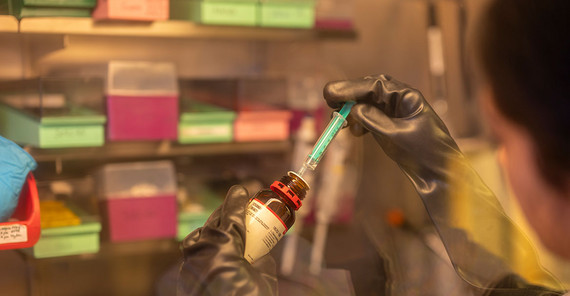
The laboratories of the Soft Matter Physics and Optoelectronics group are located on the top floor of the physics building on Golm campus. Together with two young researchers from India, we first go to the preparation laboratory. The glove boxes, from which thick black silicone gloves protrude, immediately catch the eye. In these hermetically sealed boxes, sensitive materials can be processed dust-free and in an artificial atmosphere – without oxygen and humidity. Now it is time for the so-called centrifugal coating. “We first mix organic semiconductor molecules with solvents,” PhD student Manasi Pranav explains. “We apply the solution to a 2.5 by 2.5-centimeter glass pane that is attached to a rapidly rotating plate.“ This makes it possible to cover the glass pane with a very thin semiconductor layer that is only 100 nanometers thick. By comparison, a hair is about 700 times thicker.
What a solar cell consists of
In most cases, several layers have to be stacked to build a good solar cell. The first consists of a polymer with high electrical conductivity and forms the anode of the solar cell. On top of this comes the active layer, which absorbs the light and uses it to generate free electrons and holes in the semiconductor. The final layer is a so-called electron transport layer, which ensures that the electrons generated in the active layer flow in only one direction. This enables an efficient conversion of sunlight into electric current. The metal layer is about 100 nanometers thick, collects the photogenerated electrons, and forms the cathode. It is applied in the neighboring laboratory, which contains a thermal evaporator, in which a metal is heated and vaporized by a high electric current. The metal vapors rise to the top and precipitate on the previously produced multilayer structure – and then the solar cell is ready.
The solar cell is now moved into another glove box – the characterization box. “Here we measure currents and calibrate the cell,” says Atul Shukla, who is a postdoc in Prof. Neher’s group. The light source in the box is a sunlight simulator, on which the solar cell is now mounted. Depending on the coating material, the measured voltages and currents differ, which are shown in a diagram on a screen next to the box. “In the model, we can check whether our measured values are theoretically even possible and whether the curve is compatible with the mobility of the charges,” Neher describes. He is interested in whether the result can be explained through the composition of the components.
“We know exactly what the components contain,” he says. This is a distinctive feature of the laboratory in Golm. All the individual parts of the solar cells are manufactured and analyzed here at the same time – without the group having to rely on other facilities. “We want to characterize and understand the physical processes in the solar cells in detail,” Neher emphasizes. “Our primary goal is to find overarching answers for the scientific community.” It is for good reason that Neher is one of the most cited researchers in the world, listed annually by the US company Clarivate. A good understanding of materials is important, says the physicist, who worked at the Max Planck Institute for Polymer Research in Mainz for a long time. Therefore, he attaches great importance to good cooperation within the team. “We are interested in how the different measurements and simulations of the physicists, chemists, and materials scientists fit together. To this end, we regularly network at meetings, conferences, and in our research group seminar.”
Making the cell light up
With the solar cell prototype, which was calibrated beforehand, it is now time to go to the laser laboratory – for fine characterization. On a measuring table, there is a sphere coated with white powder on the inside. The solar cell is inserted into this all-round closed light source. The sphere is connected to a spectrograph and two detectors that cover both the visible range of the spectrum and the near-infrared range. “With this, you can measure how many photons are absorbed by the solar cell and how many are emitted again,” Shukla says. The so-called quantum efficiency of photoluminescence is determined from it, an important measure of how effectively the incident light can be converted into electricity. In the same setup, the electroluminescence of the solar cell is then measured, i.e., the property of a semiconductor to emit light when voltage is applied. The three researchers put it this way: “A good solar cell must function like a good light-emitting diode.”
With other setups, the research group investigates the time scales on which the processes occur that generate free charges and ultimately an electric current from the absorption of light quanta. The conversion processes are complicated and difficult to capture, so a high time resolution of nanoseconds (1x10-9 seconds) or even a few femtoseconds (1x10-15 seconds) is necessary for this type of measurement. “With our test setups, we can cover the different time scales that determine the efficiency of the solar cells,” Prof. Neher emphasizes.
Particularly in recent years, research on organic solar cells has experienced a real boom. Many groups have now achieved efficiencies of 20% and more. Organic solar cells also have many advantages: Their production is environmentally friendly with a low CO2 footprint. They are light and flexible and therefore suitable for innovative applications. Approaches that combine organic semiconductors with other semiconductor materials in so-called tandem cells are also promising. “If you combine, for example, an organic and a perovskite solar cell in a tandem cell, the solar spectrum can be used to the maximum, thus improving efficiency,” Prof. Neher says. While organic solar cells are based on hydrocarbons, perovskites are a class of materials with the same crystal structure as the mineral CaTiO3, which is currently of interest to researchers worldwide.
With perovskite-organic tandem cells, efficiencies of 24% and more can now be easily achieved with an active layer of just one micrometer. “Electricity from photovoltaics is cost-effective but requires a lot of space. That’s why our research focuses on how to increase the efficiency of the modules,” the physicist summarizes. This would take us one step closer to a sustainable system of energy supply.
Light consists of photons or quanta, miniscule packets of energy that move at the speed of light and exhibit both wave and particle properties.
A semiconductor has qualities of both an electric conductor and a non-conductor. This includes materials like silicon and germanium. When the semiconductor is excited by applying energy, negatively charged electrons are released from the lattice and can move freely through the semiconductor, leaving behind positively charged electron holes.
Dieter Neher has been a Professor of Soft Matter Physics and Optoelectronics at the University of Potsdam since 1998.
This text was published in the university magazine Portal – One 2025 “Children” (PDF).