During embryonic development, the heart is the first organ that becomes functional and generates blood flow throughout the body. The special thing about its morphogenesis is the interconnection with the biomechanical forces due to blood flow. These forces have a big impact in particular on the cells that line the interior of the heart. From these cells, cardiac valves are formed to ensure an unidirectional flow of blood.
Zebrafish eggs can easily be manipulated and are therefore a widely used model system in biomedical research. Taking advantage of the zebrafish embryo, the researchers around Salim Seyfried combined in vivo imaging and gene expression profiling - the measurement of the activity of thousands of genes at once - to visualize the conversion of genetic information into proteins (gene expression) under conditions with and without blood flow, respectively. They observed higher Vegfr3/Flt4 gene expression in endocardial cells that lost contact to blood flow after they migrated into the area between the endocardium and the myocardium.
By studying zebrafish embryos that are defective for the Vegfr3/Flt4 gene, the team found that Vegfr3/Flt4 is essential for the proper formation of cardiac valves. Federica Fontana, first author of the paper now published, explains: „In particular, our work suggests that valve endocardial cells in the area between the endocardium and the myocardium loose expression of another gene (Notch), due to the lack of mechanical stimuli, and this triggers gene expression of Vegfr3/Flt4. In turn, Vegfr3/Flt4 suppresses the Notch-gene in these cells.” These activities represent a fine-tuned gene regulatory mechanism, essential to shape cardiac valve leaflets by inducing unique differences in the fates of endocardial cells.
Link to Publication: Federica Fontana, Timm Haack, Maria Reichenbach, Petra Knaus, Michel Puceat, Salim Abdelilah-Seyfried, 2020, Antagonistic Activities of Vegfr3/Flt4 and Notch1b Fine-tune Mechanosensitive Signaling during Zebrafish Cardiac Valvulogenesis, Cell Reports 32, 107883, https://doi.org/10.1016/j.celrep.2020.107883
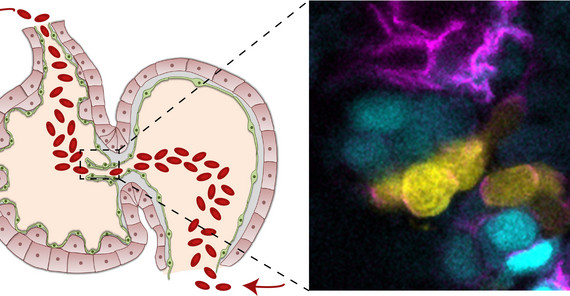
Image: In the embryonic zebrafish heart, the endocardium that is forming cardiac valve leaflets is divided into two subpopulations (here, cyan and yellow). Yellow cells are in contact with blood flow and express Notch, while Vegfr3/Flt4 is active within the cyan cells that lack blood flow stimuli. Their antagonistic activities shape the cardiac valves. Image Credit: Federica Fontana
Contact:
Prof. Dr. Salim Seyfried, Institute of Biochemistry and Biology, Tel.: +49 331 977-5540, salim.seyfrieduuni-potsdampde
Federica Fontana, Institute of Biochemistry and Biology, Tel.: +49 331 977-5539, fontanatuuni-potsdampde
Media Information 03-08-2020 / Nr. 075