Plasmons and chemistry
We study plasmonic excitations in the contexts of soft matter and light-driven chemical reactions at nanoscale metals.
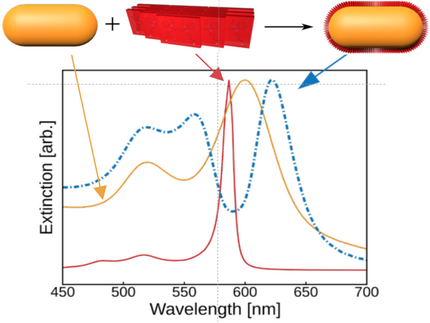
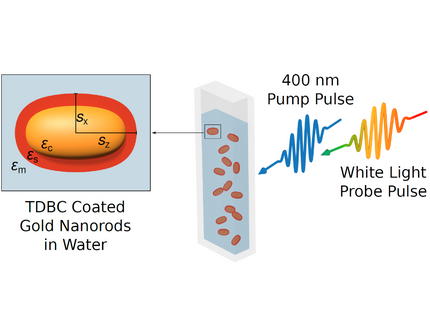
We prepare colloidal samples and investigate how the plasmon resonance can be tuned via layer-by-layer deposition of polyelectrolytes.
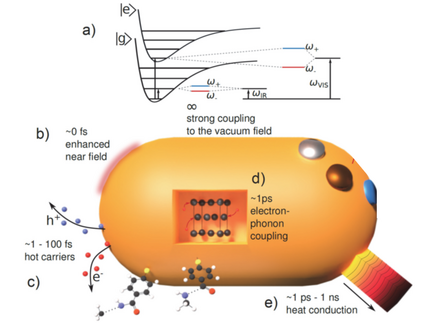
Schematic representation of the relevant fundamental primary processes occurring at nanoscale metals and a connection to the schematic representation of a catalytic cycle.
a) Even in the absence of light, the field enhancement amplifies electromagnetic vacuum fluctuations which modify potential energy surfaces by strong coupling. The timing of the other processes is essential: When a photon arrives, b) its electric field is enhanced in the near field. The LSP decays within few femtoseconds (fs) to c) hot electrons and holes, where initially a single electron or electron-hole pair carries the entire photon energy. Electrons equilibrate to a Fermi-Dirac distribution within <100 fs and d) electron-phonon coupling transfers the heat to vibrations within ~1 ps. e) The heat energy of electrons and phonons is conducted on all timescales. The secondary amide molecule sketched at the bottom is fragmented along two different pathways, depending on whether hot electrons as in c) or vibrational heat as in e) dominate.
Strong coupling
When the nanoparticles are coated with molecular layers that exhibit strong absorption at the plasmon resonance of the metallic core, a strong coupling of the excitations can result in hybrid polaritonic states that exhibit a splitting of the resonances. The localized surface plasmon polaritons can be confined to very small mode volumes of nanoparticles, such that the coupling strength exceeds all dissipation channels. This is the regime of strong coupling. A related phenomenon is the bleaching of the hybrid absorption resonances by the very intense vacuum fluctuations that are present even in the absence of light.
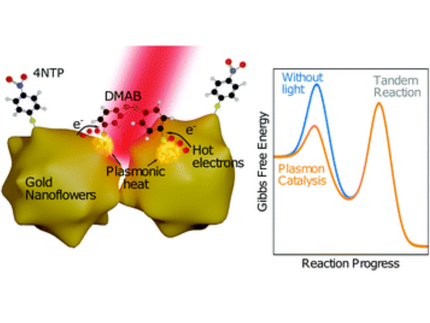
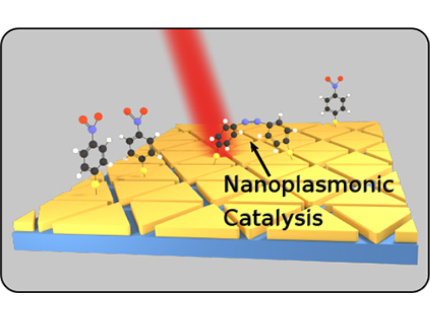
"Plasmon"-driven chemistry
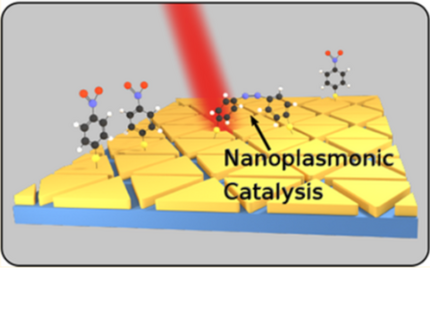
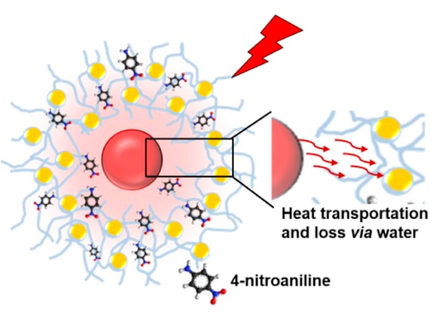
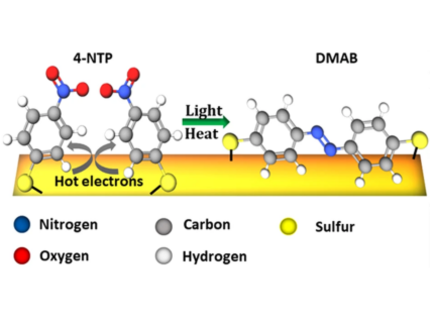
"Plasmon"-driven chemistry is an emerging field at the interface of nanophysics and photochemistry. We have focused on the dimerization of 4-Nitrothiophenol (4-NTP) to Dimercaptoazobenzene (DMAB) at the surface of various nanoscale metal geometries. It is an open question, how the plasmon-assisted generation of hot charge carriers influences this reaction in addition to non-equilibrium vibrational heat. In a broader setting, we have applied surface-enhanced Raman scattering to nanoscale metals of various shapes and compositions to demonstrate the effect of local field enhancement.
Related publications
M. Mattern, J.-E. Pudell, J. A. Arregi, J. Zlámal, R. Kalousek, V. Uhlíř, M. Rössle, and M. Bargheer
Accelerating the Laser-Induced Phase Transition in Nanostructured FeRh via Plasmonic Absorption
Advanced Functional Materials, 2313014 (2024).
By ultrafast x-ray diffraction (UXRD), it is shown that the laser-induced magnetostructural phase transition in FeRh nanoislands proceeds faster and more complete than in continuous films. An intrinsic 8 ps timescale is observed for the nucleation of ferromagnetic (FM) domains in the optically excited fraction of both types of samples. For the continuous film, the substrate-near regions are not directly exposed to light and are only slowly transformed to the FM state after heating above the transition temperature via near-equilibrium heat transport. Numerical modeling of the absorption in the investigated nanoislands reveals a strong plasmonic contribution near the FeRh/MgO interface. The larger absorption and the optical excitation of the electrons in nearly the entire volume of the nanoislands enables a rapid phase transition throughout the entire volume at the intrinsic nucleation timescale.
M. O. Adesina, M. O. Alfred, H. Seitz, K. Brennenstuhl, H. M. Rawel, P. Wessig, J. Kim, A. Wedel, W. Koopman, C. Günter, E. I. Unuabonah, and A. Taubert
Orange peel biochar/clay/titania composites: low cost, high performance, and easy-to-reuse photocatalysts for the degradation of tetracycline in water
Environmental Science: Water Research & Technology 10, 1432 (2024).
New orange peel biochar/clay/titania nanocomposites (NCs) were studied for photocatalytic degradation of tetracycline (TET) under both UV and natural solar irradiation by variation of NC dose, initial TET concentration, ionic strength, and competing anions. Total organic carbon (TOC) reduction was used to assess mineralization. Intermediate product formation during TET degradation was characterized using liquid chromatography-mass spectrometry and agar-based diffusion assays. The as-synthesized material prepared with biochar obtained at 600 °C (C600KT) exhibits the best TET degradation performance under UV light exposure and solar irradiation with up to 92 and 89% after 2 h, respectively. Especially under UV exposure, C600KT exhibits the highest apparent rate constant of 2.9 × 10−2 min−1 and a half-life of 23.9 min. About 60 and 50% TOC are removed after 2 h under UV and solar irradiation, respectively. Quenching experiments confirm that superoxide and hydroxyl radicals are the major reactive species involved in the degradation process. Furthermore, the treated effluents are harmless to both Escherichia coli and Staphylococcus xylosus, indicating that no intermediate products with higher toxicity are produced during the photocatalytic degradation. Additionally, the results show that the main fraction of TET is degraded within the first 15 min of irradiation. The C600KT composite is recyclable and retains its performance over at least four cycles, proving its stability and reusability. Overall, the new NCs are therefore highly attractive for the remediation of TET pollution in water.
Xu X., Sarhan R. M., Mei S., Kochovski Z., Koopman W., Priestley R. D., and Lu Y.
Photothermally Triggered Nanoreactors with a Tunable Catalyst Location and Catalytic Activity
ACS Applied Materials & Interfaces 15, 48623 (2023).
Thermosensitive microgels based on poly(N-isopropylacrylamide) (PNIPAm) have been widely used to create nanoreactors with controlled catalytic activity through the immobilization of metal nanoparticles (NPs). However, traditional approaches with metal NPs located only in the polymer network rely on electric heating to initiate the reaction. In this study, we developed a photothermal-responsive yolk–shell nanoreactor with a tunable location of metal NPs. The catalytic performance of these nanoreactors can be controlled by both light irradiation and conventional heating, that is, electric heating. Interestingly, the location of the catalysts had a significant impact on the reduction kinetics of the nanoreactors; catalysts in the shell exhibited higher catalytic activity compared with those in the core, under conventional heating. When subjected to light irradiation, nanoreactors with catalysts loaded in the core demonstrated improved catalytic performance compared to direct heating, while nanoreactors with catalysts in the shell exhibited relatively similar activity. We attribute this enhancement in catalytic activity to the spatial distribution of the catalysts and the localized heating within the polydopamine cores of the nanoreactors. This research presents exciting prospects for the design of innovative smart nanoreactors and efficient photothermal-assisted catalysis.
Stete F., Bargheer M., and Koopman W.
Ultrafast dynamics in plasmon–exciton core–shell systems: the role of heat
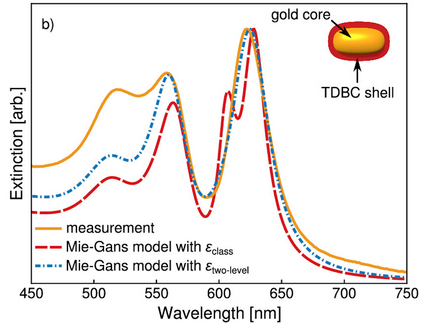
Strong coupling between plasmons and excitons gives rise to new hybrid polariton states with potential applications in various fields. Despite a plethora of research on plasmon–exciton systems, their transient behaviour is not yet fully understood. Besides Rabi oscillations in the first few femtoseconds after optical excitation, coupled systems show interesting non-linear features on the picosecond time scale. Here, we conclusively show that the source of these features is heat that is generated inside the particles. Until now, this hypothesis was only based on phenomenological arguments. We investigate the role of heat by recording the transient spectra of plasmon–exciton core–shell nanoparticles with excitation off the polariton resonance. We present analytical simulations that precisely recreate the measurements solely by assuming an initial temperature rise of the electron gas inside the particles. The simulations combine established strategies for describing uncoupled plasmonic particles with a recently published model for static spectra. The simulations are consistent for various excitation powers, confirming that heating of the particles is indeed the root of the changes in the transient signals.
Stete F., Koopman W., Henkel C., Benson O., Kewes G., and Bargheer M.
Optical Spectra of Plasmon–Exciton Core–Shell Nanoparticles: A Heuristic Quantum Approach
ACS Photonics 10, 2511 (2023).
Light–matter coupling in plasmonic nanocavities has been widely studied in the past years. Yet, for core–shell particles, popular electromagnetic models that use the classical Lorentz oscillator to describe the shell predict extinction spectra with three maxima, if the plasmon and the shell absorption are in resonance. In contrast, experiments exhibit only two peaks, as also expected from simple quantum models of hybrid states. In order to reconcile the convenient and widely used classical electromagnetic description with experimental data, we connect it to the quantum world by conceiving a heuristic quantum model. Our model is based on the permittivity of a two-level system in a classical electric field derived from the optical Bloch equations. The light–matter coupling is included via the collective vacuum Rabi frequency Ω0. Using our model, we obtain excellent agreement with a series of experimental extinction spectra of particles with various coupling strengths due to a systematic size variation. The suppression of the third maximum, which mainly stems from the absorption in the shell, can be interpreted as a vacuum induced power broadening, which may occur in lossy (plasmonic) cavities below the strong-coupling regime.
Zhao Y., Sarhan R. M., Eljarrat A., Kochovski Z., Koch C., Schmidt B., Koopman W., and Lu Y.
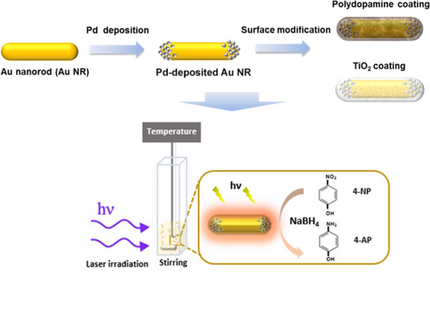
Surface-Functionalized Au–Pd Nanorods with Enhanced Photothermal Conversion and Catalytic Performance
ACS Applied Matererials & Interfaces 14, 17259 (2022).
Bimetallic nanostructures comprising plasmonic and catalytic components have recently emerged as a promising approach to generate a new type of photo-enhanced nanoreactors. Most designs however concentrate on plasmon-induced charge separation, leaving photo-generated heat as a side product. This work presents a photoreactor based on Au–Pd nanorods with an optimized photothermal conversion, which aims to effectively utilize the photo-generated heat to increase the rate of Pd-catalyzed reactions. Dumbbell-shaped Au nanorods were fabricated via a seed-mediated growth method using binary surfactants. Pd clusters were selectively grown at the tips of the Au nanorods, using the zeta potential as a new synthetic parameter to indicate the surfactant remaining on the nanorod surface. The photothermal conversion of the Au–Pd nanorods was improved with a thin layer of polydopamine (PDA) or TiO2. As a result, a 60% higher temperature increment of the dispersion compared to that for bare Au rods at the same light intensity and particle density could be achieved. The catalytic performance of the coated particles was then tested using the reduction of 4-nitrophenol as the model reaction. Under light, the PDA-coated Au–Pd nanorods exhibited an improved catalytic activity, increasing the reaction rate by a factor 3. An analysis of the activation energy confirmed the photoheating effect to be the dominant mechanism accelerating the reaction. Thus, the increased photothermal heating is responsible for the reaction acceleration. Interestingly, the same analysis shows a roughly 10% higher reaction rate for particles under illumination compared to under dark heating, possibly implying a crucial role of localized heat gradients at the particle surface. Finally, the coating thickness was identified as an essential parameter determining the photothermal conversion efficiency and the reaction acceleration.
Koopman W., Titov E., Sarhan R. M., Gaebel T., Schürmann R., Mostafa A., Kogikoski Jr. S., Milosavljević A. R., Stete F., Liebig F., Schmitt C. N. Z., Koetz J., Bald I., Saalfrank P., and Bargheer M.
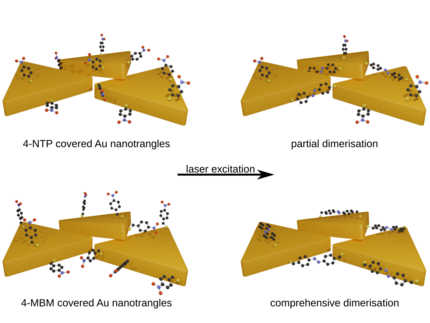
The Role of Structural Flexibility in Plasmon-Driven Coupling Reactions: Kinetic Limitations in the Dimerization of Nitro-Benzenes
Advanced Materials Interfaces, 2101244 (2021).
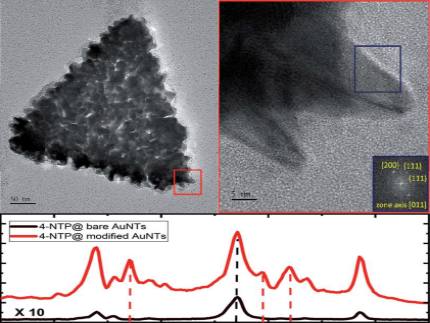
The plasmon-driven dimerization of 4-nitrothiophenol (4NTP) to 4-4′-dimercaptoazobenzene (DMAB) is a testbed for understanding bimolecular photoreactions enhanced by nanoscale metals, in particular, regarding the relevance of electron transfer and heat transfer from the metal to the molecule. By adding a methylene group between the thiol bond and the nitrophenyl, structural flexibility is added to the reactant molecule. Time-resolved surface-enhanced Raman-spectroscopy proves that this (4-nitrobenzyl)mercaptan (4NBM) molecule has a larger dimerization rate and dimerization yield than 4NTP and higher selectivity toward dimerization. X-ray photoelectron spectroscopy and density functional theory calculations show that the electron transfer prefers activation of 4NTP over 4NBM. It is concluded that the rate limiting step of this plasmonic reaction is the dimerization step, which is dramatically enhanced by the additional flexibility of the reactant. This study may serve as an example for using nanoscale metals to simultaneously provide charge carriers for bond activation and localized heat for driving bimolecular reaction steps. The molecular structure of reactants can be tuned to control the reaction kinetics.
This article is selected as "Hot Topic: Surfaces and Interfaces".
Koopman W., Sarhan R. M., Stete F., Schmitt C. N. Z., and Bargheer M.
Decoding the kinetic limitations of plasmon catalysis: the case of 4-nitrothiophenol dimerization
Plasmon-mediated chemistry presents an intriguing new approach to photocatalysis. However, the reaction enhancement mechanism is not well understood. In particular, the relative importance of plasmon-generated hot charges and photoheating is strongly debated. In this article, we evaluate the influence of microscopic photoheating on the kinetics of a model plasmon-catalyzed reaction: the light-induced 4-nitrothiophenol (4NTP) to 4,4′-dimercaptoazobenzene (DMAB) dimerization. Direct measurement of the reaction temperature by nanoparticle Raman-thermometry demonstrated that the thermal effect plays a dominant role in the kinetic limitations of this multistep reaction. At the same time, no reaction is possible by dark heating to the same temperature. This shows that plasmon nanoparticles have the unique ability to enhance several steps of complex tandem reactions simultaneously. These results provide insight into the role of hot electron and thermal effects in plasmonic catalysis of complex organic reactions, which is highly important for the ongoing development of plasmon based photosynthesis.
Liebig F., Sarhan R. M., Bargheer M., Schmitt C. N. Z., Poghosyan A. H., Shahinyan A. A., and Koetz J.
Spiked gold nanotriangles: formation, characterization and applications in surface-enhanced Raman spectroscopy and plasmon-enhanced catalysis
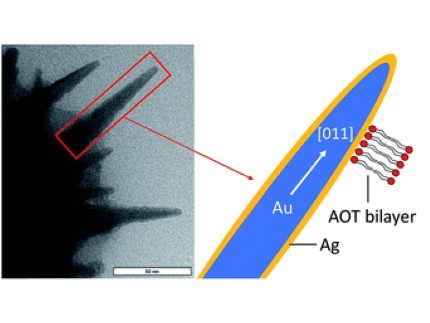
We show the formation of metallic spikes on the surface of gold nanotriangles (AuNTs) by using the same reduction process which has been used for the synthesis of gold nanostars. We confirm that silver nitrate operates as a shape-directing agent in combination with ascorbic acid as the reducing agent and investigate the mechanism by dissecting the contribution of each component, i.e., anionic surfactant dioctyl sodium sulfosuccinate (AOT), ascorbic acid (AA), and AgNO3. Molecular dynamics (MD) simulations show that AA attaches to the AOT bilayer of nanotriangles, and covers the surface of gold clusters, which is of special relevance for the spike formation process at the AuNT surface. The surface modification goes hand in hand with a change of the optical properties. The increased thickness of the triangles and a sizeable fraction of silver atoms covering the spikes lead to a blue-shift of the intense near infrared absorption of the AuNTs. The sponge-like spiky surface increases both the surface enhanced Raman scattering (SERS) cross section of the particles and the photo-catalytic activity in comparison with the unmodified triangles, which is exemplified by the plasmon-driven dimerization of 4-nitrothiophenol (4-NTP) to 4,4′-dimercaptoazobenzene (DMAB).
Liebig F., Sarhan R. M., Schmitt C. N., Thünemann A., Prietzel C., Bargheer M., and Koetz J.
Gold Nanotriangles with Crumble Topping and their Influence on Catalysis and Surface-Enhanced Raman Spectroscopy
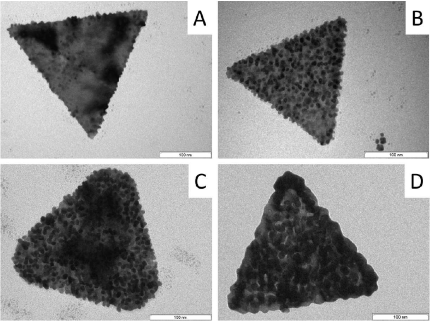
By adding hyaluronic acid (HA) to dioctyl sodium sulfosuccinate (AOT)‐stabilized gold nanotriangles (AuNTs) with an average thickness of 7.5±1 nm and an edge length of about 175±17 nm, the AOT bilayer is replaced by a polymeric HA‐layer leading to biocompatible nanoplatelets. The subsequent reduction process of tetrachloroauric acid in the HA‐shell surrounding the AuNTs leads to the formation of spherical gold nanoparticles on the platelet surface. With increasing tetrachloroauric acid concentration, the decoration with gold nanoparticles can be tuned. SAXS measurements reveal an increase of the platelet thickness up to around 14.5 nm, twice the initial value of bare AuNTs. HRTEM micrographs show welding phenomena between densely packed particles on the platelet surface, leading to a crumble formation while preserving the original crystal structure. Crumbles crystallized on top of the platelets enhance the Raman signal by a factor of around 20, and intensify the plasmon‐driven dimerization of 4‐nitrothiophenol (4‐NTP) to 4,4′‐dimercaptoazobenzene in a yield of up to 50 %. The resulting crumbled nanotriangles, with a biopolymer shell and the absorption maximum in the second window for in vivo imaging, are promising candidates for biomedical sensing.
Liebig F., Henning R., Sarhan R. M., Prietzel C., Schmitt C. N. Z., Bargheer M., and Koetz J.
A simple one-step procedure to synthesise gold nanostars in concentrated aqueous surfactant solutions
Due to the enhanced electromagnetic field at the tips of metal nanoparticles, the spiked structure of gold nanostars (AuNSs) is promising for surface-enhanced Raman scattering (SERS). Therefore, the challenge is the synthesis of well designed particles with sharp tips. The influence of different surfactants, i.e., dioctyl sodium sulfosuccinate (AOT), sodium dodecyl sulfate (SDS), and benzylhexadecyldimethylammonium chloride (BDAC), as well as the combination of surfactant mixtures on the formation of nanostars in the presence of Ag+ ions and ascorbic acid was investigated. By varying the amount of BDAC in mixed micelles the core/spike-shell morphology of the resulting AuNSs can be tuned from small cores to large ones with sharp and large spikes. The concomitant red-shift in the absorption toward the NIR region without losing the SERS enhancement enables their use for biological applications and for time-resolved spectroscopic studies of chemical reactions, which require a permanent supply with a fresh and homogeneous solution. HRTEM micrographs and energy-dispersive X-ray (EDX) experiments allow us to verify the mechanism of nanostar formation according to the silver underpotential deposition on the spike surface in combination with micelle adsorption.
Sarhan R. M., Koopman W., Pudell J.-E., Stete F., Rössle M., Herzog M., Schmitt C. N. Z., Liebig F., Koetz J., and Bargheer M.
Scaling up Nanoplasmon Catalysis: The Role of Heat DIssipation
The Journal of Physical Chemistry C 123, 9352 (2019).
Nanoscale heating by optical excitation of plasmonic nanoparticles offers a new perspective of controlling chemical reactions, where heat is not spatially uniform as in conventional macroscopic heating but strong temperature gradients exist around microscopic hot spots. In nanoplasmonics, metal particles act as a nanosource of light, heat, and energetic electrons driven by resonant excitation of their localized surface plasmon resonance. As an example of the coupling reaction of 4-nitrothiophenol into 4,4′-dimercaptoazobenzene, we show that besides the nanoscopic heat distribution at hot spots, the microscopic distribution of heat dictated by the spot size of the light focus also plays a crucial role in the design of plasmonic nanoreactors. Small sizes of laser spots enable high intensities to drive plasmon-assisted catalysis. This facilitates the observation of such reactions by surface-enhanced Raman scattering, but it challenges attempts to scale nanoplasmonic chemistry up to large areas, where the excess heat must be dissipated by one-dimensional heat transport.
Sarhan R. M., Koopman W., Schuetz R., Schmid T., Liebig F., Koetz J., and Bargheer M.
The importance of plasmonic heating for the plasmon-driven photodimerization of 4-nitrothiophenol
Scientific Reports 9, 3060 (2019).
Metal nanoparticles form potent nanoreactors, driven by the optical generation of energetic electrons and nanoscale heat. The relative influence of these two factors on nanoscale chemistry is strongly debated. This article discusses the temperature dependence of the dimerization of 4-nitrothiophenol (4-NTP) into 4,4′-dimercaptoazobenzene (DMAB) adsorbed on gold nanoflowers by Surface-Enhanced Raman Scattering (SERS). Raman thermometry shows a significant optical heating of the particles. The ratio of the Stokes and the anti-Stokes Raman signal moreover demonstrates that the molecular temperature during the reaction rises beyond the average crystal lattice temperature of the plasmonic particles. The product bands have an even higher temperature than reactant bands, which suggests that the reaction proceeds preferentially at thermal hot spots. In addition, kinetic measurements of the reaction during external heating of the reaction environment yield a considerable rise of the reaction rate with temperature. Despite this significant heating effects, a comparison of SERS spectra recorded after heating the sample by an external heater to spectra recorded after prolonged illumination shows that the reaction is strictly photo-driven. While in both cases the temperature increase is comparable, the dimerization occurs only in the presence of light. Intensity dependent measurements at fixed temperatures confirm this finding.