Summary of the research programme
Light-driven chemistry at nanoscale metals is an emerging, interdisciplinary research field. It is based on experimental and theoretical expertise ranging from nano-optics and condensed matter physics over physical chemistry to organic and inorganic chemistry. The vision is to not only control chemical reactions via the catalytic properties of nanoscale metals, but also to control and amend reaction paths so precisely that they may be activated by sunlight to enable sustainable technology. Chemical reactions that are amplified by coupling light into collective charge oscillations in the metal are known as "plasmonic chemistry", with controversial details such as the relevance of charge transfer and local heating in various reactions.
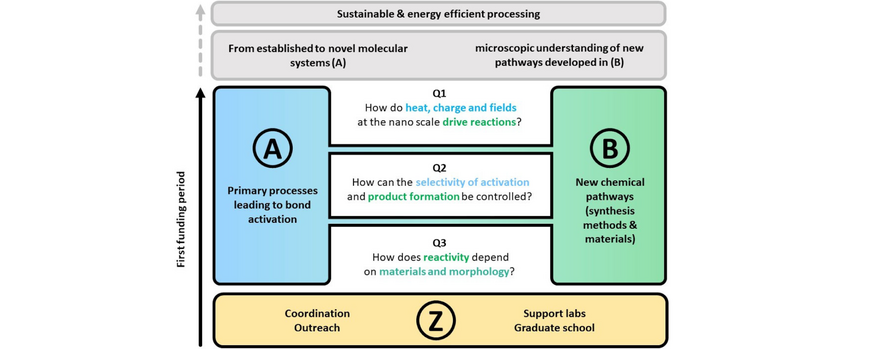
Our research programme has two goals: We want to (A) develop a comprehensive, fundamental microscopic understanding of the primary processes that lead to light-driven chemical reactions at nanoscale metals based on a set of model systems. On the other hand, we want to (B) explore new chemical pathways based on plasmon-assisted chemistry with the long-term aim to establish new materials and new synthesis methods. The research activities in (A) therefore concentrate on elementary physical processes in model systems that form the basis for new chemical reactions (B) that take place at nanoscale metals.
We focus on light-induced transformation of organic molecules, polymerisations and nanoparticle functionalization. With experiments and theoretical modelling we will investigate the elementary steps that make quantized photon energy usable for chemical reactivity: energetic electrons and holes generated by photo-excitation of the metal activate molecular bonds through charge transfer. At the same time, electronic energy is converted into vibrational excitations in the metal and its surroundings at the nanoscale. Phenomena such as hybrid light-matter states emerging through strong coupling and nanoscale heat transport including quantum effects are current challenges for experiments and modelling. Our arsenal of highly specific, ultrafast pump-probe techniques from mid-infrared to hard X-rays aims at recording the primary processes in their chronological sequence and elucidating them spectroscopically. Microscopy with atomic resolution and single molecule spectroscopy will help us to examine the reaction intermediates and products. Together we will develop and establish new plasmon-assisted synthesis schemes that enable light-induced selective and efficient chemistry. Our concentration of expertise provides an excellent opportunity to bring together researchers with strong backgrounds in chemical analysis and synthesis and characterising elementary processes. Timely interdisciplinary training across chemistry and physics will help to develop a modern view of light-driven chemistry based on modern methods of nanoscale control.
Structure of the research programme
The overarching goal of the CRC is studying the elementary processes for understanding light-driven chemistry at nanoscale metals and for developing new protocols to establish more selective, efficient and new reactions. We connect diverse research fields and develop answers to three guiding questions for light-driven chemistry at nanoscale metals.
Q1: How do heat, charge and fields at the nanoscale drive reactions?
Q2: How can the selectivity of activation and product formation be controlled?
Q3: How does reactivity depend on materials and morphology?
The following figure visualizes how the three guiding questions Q1-Q3 connect all projects and the project areas A and B. We envision that the questions and hypotheses merge the two project areas during the first funding period.