October 2025
Julie Michaud (Leibniz University Hannover) and her co-authors revisited in their new publication the tin oxidation state in a variety of samples, from the source to the deposit. Their open access paper is published in scientific reports and can be found here. The formation of so-called tin granites and their related magmatic-hydrothermal tin deposits has been studied for years and established models suggest that tin is transported in reduced granitic peraluminous magmas, exsolved from those via hydrothermal fluid and then oxidized and precipitated. However, the redox conditions are assumed and the oxidation state of tin in those magmas was never systematically analyzed. Here, Julie and her co-authors filled this gap and took samples from a broad range of relevant geological location, mainly from tin provinces of the Central Eastern Desert and the Western European Variscan belt (e.g. Erzgebirge, French Massif Central, Central Portugal) but also Mongolia and Peru. All of these specific sample locations refer to a certain step in the formation of tin deposits, such as metamorphic source rock, melt extraction, granite crystallization or fluid exsolution. The tin containing micas or bulk samples (as well as reference material) were analyzed at the DESY (Deutsches Elektronen Synchrothron) in Hamburg in order to analyze the tin oxidation state in each sample by using the Sn K-edge on the XANES spectra. These spectra reveal that tetravalent tin is predominant oxidation state in almost all samples and therefore challenging the established model as oxidation seems to occur earlier than assumed within the system. Redox reactions are not always necessary for the formation of “tin granites” or tin deposits but the data point towards the possibility that reduced conditions are a prerequisite for the formation of the largest tin deposits worldwide, e.g. San Rafael. Additionally, the study showed that tin can be used as a marker of redox conditions in a variety of geology environments, even with relatively low concentration of 30 ppm that might help solving other geological issues.
August 2025
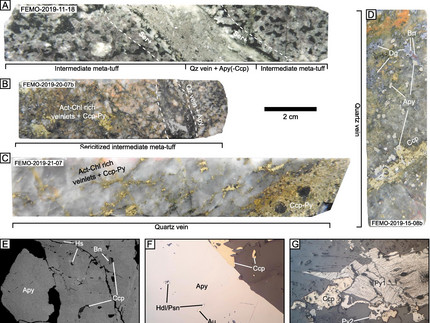
Mauro Bongiovanni (RWTH Aachen) and his co-authors continued their research on the Cornubian batholith (SW England) and investigated the behavior of the halogens in the system and how can they might be used and a fluid source indicator. The open access paper is published in Lithos can be found here. Halogens are crucial for the evolution of a magmatic system as they can influence several parameters, e.g. density, metal complexation and transport etc. (especially F and Cl) but they can also be tracers of degassing processes (Cl, Br and I). The quantification can be challenging as the halogens can be lost during ascent and cooling (especially the heavy halogens). However, due to their geochemical significance Mauro used many geochemical analytical tools to increase the precession as much as possible and to tackle the limitation of the used methods (whole rock combustion ion chromatography including leaching, EPMA, LA-ICP-MS, and fluid inclusions. The obtained data (especially halogen ratios) can serve as a robust indicator of the fluid provenance. The samples were taken on all the relevant granites (G2-G5) and related rocks of the Cornubian bathohlith, from magmatic to hydrothermal conditions. The data revealed a decreasing Cl content in the apatites and micas which indicate fluid saturation during the transition from on granitic suite to another (between G3 and G4) whereby the later also hosts the main mineralization. With their leaching procedure, they were further able to show that Br and Cl are mainly hosted by fluid inclusions or at grain boundaries and that the halogen ratios (Br/Cl, I/Cl) are not affected by boiling (as seen in the fluid inclusion data) . Additionally, they showed that the mineral budget of all the granites point towards a smooth evolution from G2 to G5 which means that the batholith underwent a continuous geochemical evolution despite the episodic nature of the granites. The fluid inclusions also revealed that later fluids have lower halogen ratios that reflect magmatic fraction and dilution with halogen free waters and not only mixing with another halogen-bearing fluid.
March 2025
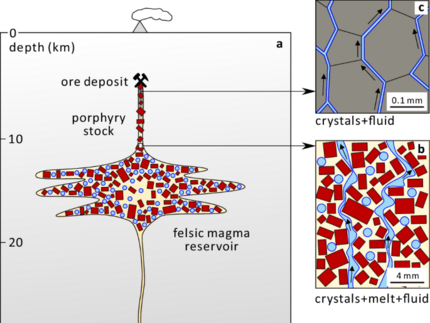
Jia Chang (former DOME Post-doc and the University of Bayreuth, currently assistant Professor in Wuhan) and his co-authors revisited the ore formation of porphyry Cu deposits to investigate where the actual mineralizing fluid origin from. Thee open access paper was published in Nature Communications and can be found here. Jia collected a whole set of evolved potassic rocks from the Sanjiang region ranging from mafic lavas, monzogranite, syenite porphyry to rhyolite tuff. With this various sample set, the authors studied the melt and fluid inclusion and combined the results (e.g. volatile and metal contents before and after fluid exsolution) with thermobarometry, therefore getting rather precise estimation of the pressure and temperature regime of the magmatic system. All in all, 207 melt inclusion in phenocrysts embedded in various minerals (depending on the sample origin varying from olivine to quartz) were analyzed with LA-ICP-MS. The inclusion hosted in mafic and intermediate rocks show trace element contents in line with previously conducted experiments at 1.0 GPa although CU,S and Au contents are slightly lower in intermediate rocks due to their compatibility to sulfides. Lower contents of the metals and volatiles in the melt inclusion of the felsic rocks represent the onset of aqueous exsolution. Different thermobarometry methods were used but all show overlapping results for the porphyry phenocrysts with mode pressures form 0.3 GPa to 0.5 GPa (representing 10-20 km). The extraction of fluids therefore occurred before the magmas finally ascend to form the porphyry stocks. Although the classical model ff porphyry copper formation already predicted big upper crustal magma reservoirs, the study by Jia showed that (at least for the Sanjiang region) this reservoir was located in depths between 10 to 20 km whereas the mineralization occurred in 5 km depth. Therefore, most the fluid had to travel ca. 10 km from the source to the site of mineralization after the emplacement of the porphyry stock (also evidenced by mass calculations) via an interconnected fluid network at near solidus conditions.
October 2024
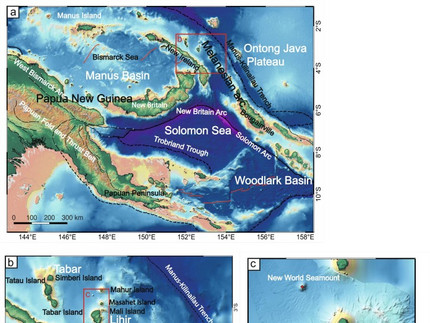
Louis-Maxime Gatreau (GEOMAR Kiel) and his co-authors published their paper "Understanding the links between volcanic systems and epithermal ore formation: A case study from Conical Seamount, Papua New Guinea" in Lithos. The full open-access paper can be found here. Their study investigates the relationship between volcanic activity and the formation of epithermal ore deposits, focusing on the Conical Seamount in the Tabar-Lihir-Tanga-Feni (TLTF) island chain. This region, known for its gold and copper mineralization, provides insights into how volcanic magmatic systems contribute to the development of ore-forming processes. The samples were collected during several cruises from 1994 until 2002 and were mainly analyzed via whole rock geochemistry, EPMA, trace element modelling, and melt inclusions. The key finding of the study is that the Conical Seamount stands out from other nearby volcanic systems like Edison, Tubaf, and New World due to the presence of a shallow magma reservoir. While all these seamounts are underpinned by similar mantle sources, Conical Seamount’s shallow magma chamber undergoes frequent replenishment, which causes significant magma fractionation. This process leads to sulfide saturation and the exsolution of volatiles, which in turn enriches the magma with chalcophile elements, including gold. As a result, metals are transferred into the overlying hydrothermal system, contributing to the formation of the epithermal ores found in the area. The study suggests that the volcanic system at Conical Seamount operates through a two-stage melting process. The first stage leaves the mantle enriched in gold and other metals, and in the second stage, further melting concentrates these metals. Additionally, the shallow magma chamber plays a pivotal role in pre-enriching the magma with both metals and volatiles, which are essential for ore-forming processes. Volatile exsolution during sulfide saturation is especially important because it facilitates the transfer of metals from the magmatic system to the hydrothermal environment.
June 2024
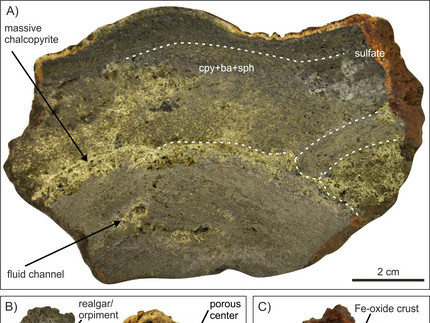
The article "The Spatial and Temporal Evolution of the Sadisdorf Li-Sn-(W-Cu) Magmatic-Hydrothermal Greisen and Vein System, Eastern Erzgebirge, Germany", authored by Dino Leopardi of the Helmholtz-Zentrum Dresden-Rossendorf and his co-authors, explores the complex magmatic-hydrothermal processes behind the formation of the Sadisdorf deposit. The open access article is published in Economic Geology and can be found here. Located in the Eastern Erzgebirge of Germany, this deposit is characterized by its association with a fractionated alkali-feldspar granite porphyry, emplaced during the late stages of the Variscan orogeny. Dino and his co authors show that the mineralization in Sadisdorf occurs in two primary forms: metasomatic greisen and vein or breccia-style mineralization. The deposit exhibits clear mineral zoning. Closest to the magmatic source is a proximal oxide-dominated zone featuring cassiterite, wolframite, and molybdenite. This transitions into a mixed zone where cassiterite coexists with Cu-Zn-Pb-As sulfides, and finally, into a distal sulfide-dominated zone containing chalcopyrite, sphalerite, galena, and arsenopyrite. Fluid inclusion analyses in quartz, cassiterite, and sphalerite suggest homogenization temperatures of 250°–418°C, with salinities ranging from 0.0 to 10.0 wt% NaCl equivalent for the mineralization. This indicates that cooling did not play a primary role in zonation, which is instead attributed to fluid-rock interactions along fluid pathways. In contrast, their analyzed fluid inclusions in late-stage fluorite veins show lower homogenization temperatures (120°–270°C) and salinities (0.0–3.0 wt% NaCl equivalent), indicative of cooling and possible meteoric water mixing. Sadisdorf shares features with other Erzgebirge deposits, such as shallow emplacement, similar mineralization styles, early fluid boiling, fluid-rock interaction, and late-stage cooling. However, it uniquely preserves both proximal and distal mineralization, providing an opportunity to study the spatial zonation in detail. This zonation is proposed as a potential exploration guide for greisen-related lithium and tin resources.
April 2024
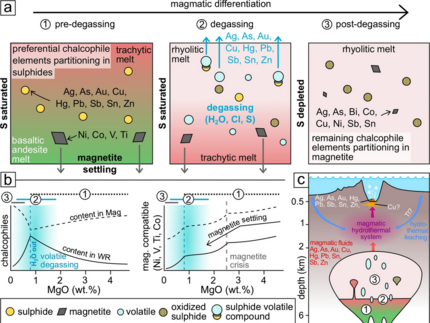
Simon Hector, DOME researcher of the Karlsruhe Institute of Technology, and his co-authors analyzed samples from the Kolombo submarine volcano located in the southern Aegean Sea and were able to describe the “Magmatic evolution of the Kolumbo submarine volcano and its implication to seafloor massive sulfide formation” in Mineralium Deposita. Their paper can be found here. Analyzing these modern ore-forming equivalents to ancient mineral deposits is crucial for understanding the mineralizing processes and can provide useful models for the exploration of massive sulfides. A special aspect of the paper is the described arc-related hydrothermal system which differs from other well-studied mid-ocean ridge systems (especially their metal content but also fluid flow, precipitation mechanism, etc.) as arc-related systems show similarities to on-land porphyry systems. The samples were taken by an expedition done in 2010 and later drilling during the IOPD (International Ocean Discovery Program) expedition. The samples were mainly analyzed via microscopy (reflected and transmitted light), whole rock geochemistry, and EPMA with special emphasis on the magnetite composition. Further, Simon and his co-authors used COMAGMAT 3.75 to model the evolution of the melt and get insights into the metal mobilizing processes. Using these techniques, the authors show that, although early magmatic sulfide saturation is occurring, the magmatic sulfide (and also the chalcophile elements) do not fractionate. However, once water saturation is reached and degassing is starting, many elements (e.g. As, Ag, Cu, Sn, Pn, Zn) are mobilized and are partitioned into the volatile phase directly out of magma or by sulfide oxidation. The leftover chalcophile elements are incorporated into the magnetite which was the key mineral to track these processes.
November 2023
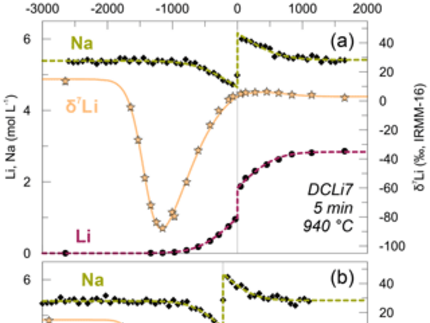
Christian Singer, a DOME researcher at the University of Hannover, and his co-authors published their work on the “Li-Na interdiffusion and diffusion-driven lithium isotope fractionation in pegmatitic melts” in the European Journal of Mineralogy. The open acess paper can be found here. The transport mechanism of rare elements and how they are enriched in pegmatites is still highly debated and not well-constrained. Although previous work evaluated Li diffusion in various magmatic rocks, this study shows the first experimental results of Li diffusion in pegmatitic melts using experimental conditions ranging from 650 to 940°C at 100 MPa. Pegmatites and their large crystals form especially due to the influence of fluxes (e.g. Li, B, P, and F) which can lead to decreasing liquidus temperature and melt viscosity. Bringing this together, Christian further investigates the influence of these fluxes on the Li diffusivity and the different Li isotopes. The data shows diffusion profiles of a total length of 2000 µm of Li and Na around the contact of the produced synthetic glass cylinders (one with Li and one Li-free and pegmatitic composition). Further, it becomes evident that the lighter 6Li diffuses faster than the heavier 7Li. The diffusion of the elements between the glasses can already be observed in relatively short experimental setups (1-30 min max.) The calculated Li-Na interdiffusion coefficient is in agreement with studies done with other dry aluminosilicate melts and therefore confirms the little effect of the aluminosilicate melt composition and the added fluxes to the diffusivity.
October 2023
Dino Leopardi, DOME researcher from the HZDR/TU Freiberg, and his co-authors published a paper in Geochemistry in which they present amazing age data obtained from cassiterites within the Sadisdorf mineralization located in the Erzgebirge. The open-access paper “LA-ICP-MS U-Pb cassiterite age data of the Sadisdorf deposit link Sn-Li-(W-Cu) mineralization in the eastern Erzgebirge to the collapse of the Altenberg-Teplice Caldera” can be found here. The Sadisdorf prospect consists of four intrusive stages of which the youngest alkali-feldspar microgranite porphyry hosts relevant mineralizations. The prospect is divided into two sites, one centered on the microgranite (Kupfergrube) with greisen and stockwork mineralization within the endo- and exocontact and one to the SE with vein and stockwork-style mineralization (Zinnklüfte) albeit fewer ore minerals. Cassiterite and wolframite occur disseminated within the massive quartz-topaz-mica greisen bodies whereas the stockwork style mineralization shows quartz-mica-topaz veinlets with early Sn and W oxide and late sulfides. Although previously inferred to be related to two distinct mineralizing events, Dino and his Co-authors present a rather narrow time range for both types. Their results are based on 16 samples from the Sadisdorf system which allowed a more detailed evaluation compared to the previous investigation. With this approach, it was possible to evaluate the age consistency and further contextualize the timing of ore formation within the regional/province scale. 20 unaltered domains within these cassiterites were chosen resulting in in-situ dates of the Kupfergrube and Zinnklüfte sites ranging from 311 to 315 Ma but overlapping in general. This points towards the mentioned coeval formation and relation to one magmatic-hydrothermal event.
July 2023
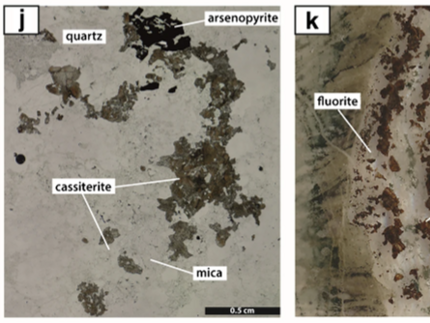
The new paper by Nicolas Meyer (DOME researcher at the University of Tübingen) gives an amazing insight into the evolution of a magmatic-hydrothermal skarn vein deposit in the Erzgebirge. He and his co-authors published their research in Mineralium Deposita with the title “Timing and origin of skarn‑, greisen‑, and vein‑hosted tin mineralization at Geyer, Erzgebirge (Germany) “. The Geyer mining district is located in the western part of the Erzgebirge and its mineralization comprises different styles of mineralization including tin-bearing skarns, greisen, and veins in which the cassiterite is the main ore mineral. The authors provide a detailed petrographic description of the ore deposit and are further using fluid inclusions microthermometry and LA-ICP-MS geochronology to decipher the formation of the deposit. The microscopic analysis of a set of 60 samples already provides a paragenetic sequence that points towards two formation stages of which the first stage is a skarn alteration with no tin occurrence. The second magmatic stage consists of a complex skarn and greisen alteration followed by late-stage veins. Cassiterite is present in all these different mineralizing events. These petrographic observations are in line with the U-Pb age dating results made by Nicolas and his co-authors. The obtained ages in garnet clearly show these two stages with ages of ~322 Ma for the first stage and 307 to 301 Ma for the garnet in the second stage supported by cassiterite ages of 308 to 305 Ma preserved in the veins. This points towards a common magmatic-hydrothermal event responsible for the ore-forming event. Within this second stage, the tin is mainly precipitated by cooling and dilution of the magmatic-hydrothermal fluid by meteoric fluids as shown by the fluid inclusion data.
May 2023
DOME researcher Dominic Gudelius and his co-authors published a paper in the Journal of Petrology and compared alkaline complexes in Greenland (Gardiner) and Russia (Kovdor). In these alkaline complexes, ultramafic rocks can occur together with carbonatites and melilitolites and can contain different types of HFSE (high-field strength element) mineralization. Therefore, Gudelius et al. analyzed these rather similar successions of rocks but found evidence that the formation can be rather different. In the Gardiner complex, the ultramafic rocks originate from primitive mantle-derived melts and therefore show e.g. cumulate textures or Ni-rich forsterite. The parental melts in Gardiner were extracted from a weakly oxidized (Sr-, F-, Ti-rich) mantle and experienced long-lasting differentiation therefore forming the carbonatites, foidolites, etc. The ultramafic rocks of the Kovdor complex are the product of metasomatic interaction between dolomitic mantle-derived carbonatite magma and diverse crustal host rocks. Here, no cumulate structures can be found. However, a subsuite of samples of Kovdor shows these cumulate structures pointing towards the possibility that these two processes (cumulate-derived ultramafic rock formation and formation by carbonate metasomatism) can happen simultaneously. The results of the paper further indicate that the parental magmas should derive from a Ti (+HFSE) enriched mantel to form HFSE enrichments early in the magma evolution as well and high F contents in the melt facilitate HFSE mobilization. Depending on the magma evolution, carbonatites can be enriched or depleted in the late-stage carbonatites. Therefore, a characterization of the ultramafic rocks in these alkaline complexes is critical for exploration, as HFSE mineralization is either hosted by the ultramafic rocks or the carbonatites.
March 2023
Simon Hector (Karlsruhe Institute of Technology, KIT) and his co-authors analyzed four Au deposits with varying base metal content in their paper “Orogenic Au deposits with atypical metal association (Cu, Co, Ni): Insights from the Pohjanmaa Belt, western Finland” published in Ore Geology Reviews. The open-access paper can be found here. These orogenic Au deposits are typically epigenetic, hosted in greenstone belts and usually only contain Au as a commodity but some show economic enrichments of Co, Ni, and Cu. These deposits are then called orogenic Au deposits with atypical metal associations. The occurrence of both, Au deposits with and without atypical metal association, within the same geological structures raises questions regarding the relative timing of the mineralization and the source and nature of the fluid. Simon analyzed a big set of samples from the four deposits and investigated the mineralogy by microscopy (including transmitted and reflected light), SEM, and EPMA. Additionally, he studied the sulfur isotopes of monomineralic powders of sulfides within the samples. The evaluation of the data showed that there were multiple successive mineralizing events of which two are the main Au mineralizing events. The first event is related to the peak metamorphism in which Au occurs in arsenides (invisible gold) or as inclusion in arsenopyrites. The formation temperatures are between 620 and 430°C and this event can further bring in Co and Ni. The later fluid event can form Cu (-Au) rich sulfide and is related to the retrograde overprint. Here, the Au occurs as free, native grains. The relative intensity of each mineralizing event is then responsible for the occurrence of either Au or Au-Cu, Au-Co, and Au-Cu-Co deposits. Altough these atypical Au deposits are sometimes classified as porphyry copper deposits overprinted by orogenic Au mineralization, Simon showed that the results of his study are not consistent with features observed in porphyries, especially as the Cu mineralizing event postdates the Au-As formation.
January 2023
Jia Chang (University of Bayreuth) published his results regarding the “Post-subduction porphyry Cu magmas in the Sanjiang region of southwestern China formed by fractionation of lithospheric mantle-derived mafic magmas” in Geology. In his paper, he replaces the popular model that post-subduction porphyries are formed by partial melting of lower crustal cumulates with a new model in which these deposits are formed by fractionation of mafic magmas derived from the subduction-modified lithospheric mantle. This can also explain the K-rich nature of these porphyries whereas arc cumulates and derived partial melts are K-poor. He undertook an invasive petrographic study and produced new Nd-Sr isotopic and trace element data from xenoliths with varying degrees of metasomatism. He and his co-author display the measured trace element contents (especially La/Yb) and link those to the collision front. It becomes clear that although the La/Yb ratios are constant over the mafic to felsic interval at one location, the ratio increases with distance to the collision front in the analyzed mafic, intermediate, and felsic rocks. Therefore, the post-subduction porphyries are more likely to directly derive from the associated mafic magmas. Furthermore, the constant values in individual magma systems points against the crustal melting model as different degrees of partial melting would also result in highly variable La/Yb ratios which are not observed.
March 2022
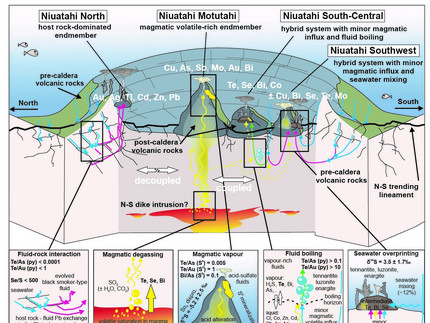
Jan Falkenberg from the GeoZentrumNordbayern published his next paper regarding the "Spatial Variations in Magmatic Volatile Influx and Fluid Boiling in the Submarine Hydrothermal Systems of Niuatahi Caldera, Tonga Rear-Arc" in Geochemistry, Geophysics, Geosystems. His open access article can be found at here. It shows really well that seafloor mineralization (e.g., black smokers) with different compositions can occur at hot springs at submarine caldera volcanoes associated with volcanic cones or the caldera wall. The effect of boiling fluids and the addition of magmatic gases results in local differences in the metal budget in the related hydrothermal sulfide minerals, which are poorly constrained. The Niuatahi volcano in the western Pacific is such an example, where four different hot springs are discharging up to 334°C hot fluids with variable salt and metal contents. The trace element and isotope composition of hydrothermal sulfide minerals agree with the hot fluid composition and indicate that fluid boiling at the caldera center is a common process, alongside the influx of magmatic gases, which drastically enhances the metal budget of the mineralization. By contrast, metal transfer due to magmatic gas is not evident in the hot springs at the northern caldera wall, which is rather controlled by evolved seawater and fluid interaction with the surrounding rocks. The observed chemical variations show that Niuatahi caldera is host to a continuum from magmatic gas-rich to host rock-controlled hydrothermal systems, which ultimately results in seafloor mineralization that exhibit spatially selective trace element enrichments.
February 2022
Dominik Gudelius, DOME researcher from the University of Tübingen, published his article “Crustal fluids cause strong Lu-Hf fractionation and Hf-Nd-Li isotopic provinciality in the mantle of continental subduction zones” in Geology. The link can be found here. His work shows how the terrestrial element cycles can be better understood via the analysis of various trace elements and their isotopic signature (including Lu-Hf, Li-Nd) in metasomized mantle peridotites. He and his co-authors sampled such peridotites within exhumed high-pressure terranes, like the high-grade gneisses of the Ulten Zone in the Alps (see figure for an overview of the diverse peridotite structures). This work shows really well the complexity, the sensitivity and the various links of the isotopic distribution to the undergone fluid-rock interaction and the mineralogical reactions. These reactions are responsible for a variety of element losses/gains including heavy rare earth element loss (HREE), high field strength element (HFSE) addition and light rare earth element fractionation. The authors suggest that the observed heterogeneity in the peridotites of the Ulten Zone are caused by their different position relative to the crustal fluid source.
December 2021
Maximilian Korges and his co-authors showed in their study the mineralogical change of the Merensky Reef, Eastern Bushveld, South Africa during weathering processes. The supergene processes resulted in an invasive change of the mineralogy of the Merensky Reef, including the PGM (platin group minerals). Whole-rock geochemistry data showed the (still) high contents of PGE (platinum group elements), similar to the content in pristine samples of the Merensky Reef. However, the processing of this supergene ore is uneconomic. Therefore, Korges et al. investigated the mineralogical sitting of the PGE as there are ca. hundreds of million tonnes of supergene ore worldwide than cannot be processed using conventional methods although showing this high PGE contents. It became clear that the distribution of PGE within the supergene ores change from bimodal in the pristine to a polymodal distribution. PGE in the pristine ores are mainly detectable as discrete minerals (PGM) mostly attached to sulfides and further within the crystal structure of sulfides (mainly Pd in pentlandite). In the supergene, they found relict PGM and "neoformation" (typically alloys) which were not present in the pristine samples. Further, relict sulfides as well as their weathering product (e.g. Fe-hydroxides) can still contain high grades of Pd. Further, some hydro-silicates can show elevated contents of PGE although the mineralogical sitting within silicates remains unclear. In the end, the microscopical investigation and the lack of, especially, Pt detected in the secondary phases and/or as PGM suggest the formation of submicrometer/nanometer-sized Pt minerals during weathering which have to be processed via whole-rock leaching processes.
November 2021
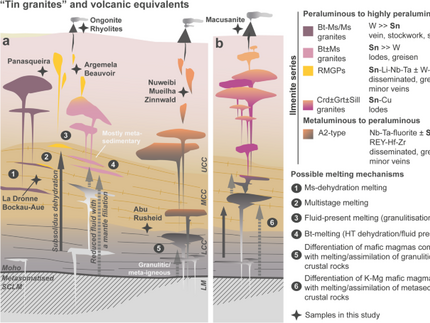
Julie Michaud (DOME researcher at the University of Hannover) published an article in Constributions to Mineralogy and Petrology . She and her co-authors decribe the behavior of different rare elements in peraluminous granites wih several experiments. Here you can find the abstract of the article.
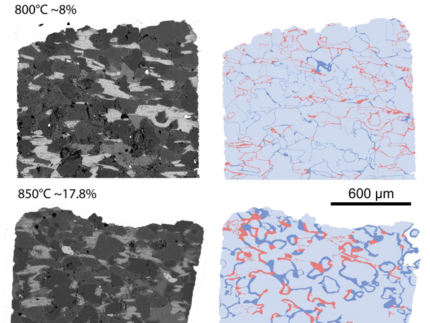
Experiments were conducted to explore the behavior of Li, Rb, Nb, Sn, Cs, Ta, W during crustal melting and test the anatectic origin of rare metal-bearing peraluminous granites such as rare metal granites (RMGs). The experiments were performed under fluid-absent conditions at 800 and 850 °C, 400 MPa and moderately reducing fO2 (ΔFMQ = − 0.5 to − 0.8). Starting materials were cores of several millimetres drilled from two natural rocks, a biotite-rich paragneiss (Pg) and a muscoviterich orthogneiss (Og) enriched in Li, Be, Sn, Cs, W. Both protoliths produced small melt fractions from 8 to 20% vol. Melt distributions were either homogeneously distributed at grain boundaries in the Pg or preferentially associated with muscovite reaction zones in the Og. In the Pg at 800 °C, melting is mainly fluid present, driven by interstitial water at grain boundaries. At 850 °C, biotite dehydration-melting produces peritectic orthopyroxene, hercynitic spinel, ilmenite and alkali feldspar in addition to melt. In the Og, muscovite dehydration-melting generates melt plus peritectic biotite, hercynitic spinel, ilmenite, Al silicates and alkali feldspar. Experimental glasses are nearly homogeneous, silica rich, peraluminous and leucogranitic and their major element compositions differ only little between the two protoliths. In contrast, the trace element concentrations vary as a consequence of chemical and textural heterogeneities in our starting materials. Compared with source rocks, the Og glasses are enriched in Rb, Nb, Ta, W and depleted in Li, Cs and the Pg are enriched in Li, Rb, Cs, W and depleted in Nb, Ta. Mass-balance calculations indicate that during muscovite dehydration-melting, Li, Cs and Rb partition into the melt; whereas Nb, Ta and W are preferentially incorporated in peritectic phases. Li and Cs also partition toward the melt during biotite dehydration-melting. The partitioning behavior of trace elements during crustal melting is a function of the melting reaction and partition coefficients between melt, residual and peritectic phases. Experimental glasses are similar to peraluminous muscovite granites but fail to reproduce RMG compositions. Alternatives to mica dehydration-melting such as fluid-present and residual source melting emphasize the difficulties with an origin of RMGs by purely anatectic processes. Crystallization differentiation might have to be combined with mica dehydration-melting to explain the distinctive geochemical features of RMGs.
June 2021
Jan Falkenberg (DOME researcher at the GeoZentrum Nordbayern) published an article about the "Effects of fluid boiling on Au and volatile element enrichment in submarine arc-related hydrothermal systems". Jan and his co-authors analyzed samples from the black-smoker chimneys and conclude from their bulk sulfide-sulfate, isotope and microanalytical data that volatile (e.g., As, Sb, Se, Te), and precious (Au) element enrichment in submarine arc-related hydrothermal systems can be decoupled from a magmatic volatile influx and is instead a result of boiling-induced trace element fractionation. Here the abstract of the paper:
Shallow (<1500 mbsl) submarine arc-related hydrothermal systems can host base (Cu), precious (Au) and volatile elements (As, Se, Sb, Te, Tl) in significant quantities. Their wide application in the high-tech industry, but a potential eco-toxicological footprint gives them a strategic importance. However, the processes that concentrate these elements in submarine arc-related hydrothermal systems, compared to their mid-ocean ridge counterparts are still debated, and it is unclear whether boilingrelated processes and/or the contribution of magmatic volatiles are key for their enrichment.
We present bulk sulfide-sulfate, isotope (S and Pb), and high-resolution microanalytical data of hydrothermal sulfides from the Niua South fore-arc volcano in north Tonga, where numerous black-smoker type sulfide-sulfate chimneys emit boiling fluids with temperatures (up to 325 _C) near the seawater boiling curve at ~1170 m water depth. Hence, this system represents an ideal natural laboratory to investigate the effect of fluid boiling on base, precious, and volatile element enrichment associated with hydrothermal seafloor mineralization. At Niua South, textural and chemical variations of multiple pyrite (framboidal, euhedral and massive), chalcopyrite (linings), and sphalerite (dendrites and linings) generations are indicative for sulfide precipitation from early low-temperature (~240 _C) fluids that underwent abundant mixing with ambient seawater (low Se/Tl and Co/Ni ratios in pyrite) and from later high-temperature (up to 325 _C) (high Se/Tl and Co/Ni ratios in pyrite). In addition, crustiform inclusion-rich pyrite that precipitated from high-temperature boiling fluids shows low Bi/Pb, Tl/Pb and Sb/Pb ratios due to volatile element loss (e.g., Tl and Sb) to the vapor phase compared to pyrite that formed during the low temperature stage. By contrast, late sphalerite (~280 _C) is enriched in elements with an affinity to Cl-complexes like Mn, Co, Ni, Ga, Cd, In, and Sn, and therefore precipitated from the corresponding Cl-rich liquid phase.
Gold occurs in solid-solution and as boiling-induced particles of native Au, electrum, and Au-rich Bi-tellurides in pyrite (up to 144 ppm Au), sphalerite (up to 60 ppm Au), and chalcopyrite (up to 37 ppm Au). These particles (<5–10 mm) probably formed during fluid boiling causing an extreme Au enrichment (>30 ppm) in the mature and late stage of chimney formation. Lead isotope data indicate that the hydrothermal fluids scavenged metals not only from the deeper basement in the reaction zone (20–40%), but also from young dacitic volcanic rocks near the seafloor in the upflow zone (60–80%). Sulfur isotope (d34S = _0.3 to 4.4‰) and Se/S*106 values (<1500) of hydrothermal sulfides provide no evidence for a magmatic volatile influx and indicate that S, and most metals and semi-metals were likely leached from the host rocks. Hence, volatile (As, Se, Sb, Te, Tl), and precious (Au) element enrichments in arc-related submarine hydrothermal systems can be decoupled from magmatic volatiles and are instead a result of boiling-induced trace element fractionation – a hydrothermal enrichment process, which has been underestimated to date.
April 2021
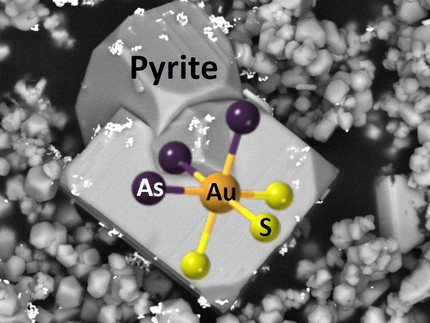
An international team of scientists with participation of Dr. Maria Kokh from one of the DOME projects was able to elucidate the mystery of ‘invisible’ gold enriched in sulfide minerals. Their results were recently published in the journal Geochemical Perspectives Letters.
To form an ore deposit, gold needs to be concentrated from a thousand up to a million times more than its average abundance in the Earth's crust (which is only about 1 mg per ton of rock). In nature, only very few minerals, namely the sulfides arsenian pyrite and arsenopyrite, are known to present such enrichment factors for gold. However, despite the enormous implications for ore deposits, the state of this ‘invisible' gold and the cause of its entrapment in these sulfides remain one of the greatest mysteries in the history of the study of ore deposits. An international interdisciplinary consortium of scientists has now shown the exact nature of this invisible gold incorporated by these minerals and revealed the fundamental mechanism that drives these ‘mineral pumps’ at the atomic-scale.
By combining high-resolution experiments – carried out at the European Synchrotron facility (ESRF) – and physical-chemical modeling, the team has discovered that gold enters these minerals with a formal oxidation state of +2. This is made possible by the occurrence of a redox reaction between the fluid and mineral that allows binding gold to arsenic, leading to the formation of the atomic cluster AuAsnS6-n in the mineral structure (Image). This universal gold-arsenic coupling mechanism explains how these iron sulfides can massively capture gold and release it later, controlling both concentration and distribution of gold in different types of hydrothermal deposits. This novel conceptual model opens perspectives for finding new sources of gold and other precious and critical metals hidden in iron sulfide minerals, and for improving the processing and recycling of metal ores for our ‘metal-hungry’ society.
Link to Publication: G.S. Pokrovski, C. Escoda, M. Blanchard, D. Testemale, J-L. Hazemann, S. Gouy, M.A. Kokh, M-C. Boiron, F. de Parseval, T. Aigouy, L. Menjot, P. de Parseval, O. Proux, M. Rovezzi, D. Béziat, S. Salvi, K. Kouzmanov, T. Bartsch, R. Pöttgen, T. Doert (2021) An arsenic-driven pump for invisible gold in hydrothermal systems. Geochemical Perspectives Letters (2021) 17, 39-44, https://doi.org/10.7185/geochemlet.2112