Articles
W. Zhao, Á. Rodríguez Echarri, A. Eljarrat, H. C. Nerl, T. Kiel, B. Haas, H. Halim, Y. Lu, C. T. Koch, and K. Busch
Time-domain study of surface plasmon polariton propagation in silver nanowires
Physical Review B 113, 085425 (2026)
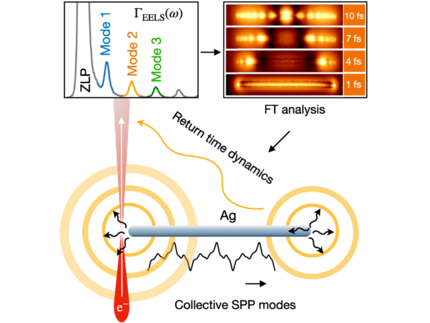
Electron microscopy techniques such as electron energy-loss spectroscopy (EELS) facilitate the spatiospectral characterization of plasmonic nanostructures. In this work, a time-dependent perspective is presented that significantly enhances the utility of EELS. In particular, this approach facilitates the analysis of the dynamics of plasmonic excitations that repeatedly interact with swift electrons in a STEM-EELS configuration. This includes the bulk plasmon mode, which can only be excited by penetrating electron beams, and the fundamental surface plasmon polariton modes propagating along the wire, which can be excited by both penetrating and aloof trajectories. In addition, the role of higher-order azimuthal surface plasmon polariton modes, often overlooked for very thin wires, is observed and analyzed in both the energy-loss spectrum and from the dynamical perspective. Such a complete understanding of the interaction of electrons and plasmonic excitations is key for the design of efficient plasmonic sensors, the study of hot electron dynamics in metals, and applications in the context of electron quantum optics, where full control of the spatial and temporal characteristics of the fields at the nanometer and femtosecond scales is highly desirable.
L. Cordsmeier, W. Ribeiro da Silva Neto, M. Fondell, R. Mitzner, V. Vaz da Cruz, S. Eckert, and A. Föhlisch
Mechanisms of Hydroxyl Radical Chemistry in Aqueous Solution Triggered by Photoexcitation and Probed by Soft X-rays
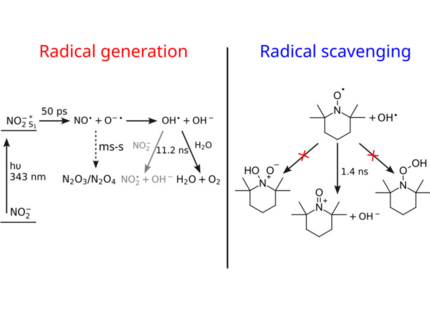
Hydroxyl radicals are among the most important radicals on earth, being present in the human body, the atmosphere, rivers, and oceans, contributing to mechanisms like oxidative stress in cells and the photochemistry of the troposphere, and posing a threat to aquatic life. Extensive use of fertilizers in agriculture has led to increased levels of nitrogen oxides in many rivers around the world, which are a major source of hydroxyl radicals in water. In this paper, we explore the photoinduced generation of hydroxyl radicals from nitrite and their scavenging by the radical scavenger 2,2,6,6-tetramethylpiperidinyloxyl (TEMPO) in aqueous solutions using transient soft X-ray absorption spectroscopy (XAS) at the oxygen and nitrogen K-edges. We show the photoinduced generation of hydroxyl radicals from nitrite and determine its mechanism. For the scavenging of hydroxyl radicals by TEMPO, we show that the mechanism does not proceed through a bound intermediate state between the two molecules, as has been proposed in the literature, but instead through an electron transfer.
M. Monai, W. Albrecht, A. Alkemper, N. Artrith, A. Baldi, A. Beck, R. T. Berry, E. Bianco, F. A. Brzesowsky, Q. Dong, J. Faria Albanese, R. R. Frontiera, E. Galvin, E. C. Garnett, N. Gerrits, M. Grzelczak, M. Herzog, F. Hess, A. A. Kolganov, W. Koopman, N. Kosinov, S. Lander, E. Lepre, D. N. Maaskant, G. Miao, A. M. Naik, T. M. Onn, A. A. Peterson, D. Piankova, E. A. Pidko, K. Trangwachirachai, F. van den Bosch, D. Xu, B. Yilmaz, J. Zeininger, E. Alarcón Lladó, J. Meyer, P. J. Dauenhauer, S. H. C. Askes
Grand Challenges and Opportunities in Stimulated Dynamic and Resonant Catalysis
ACS Catalysis XX, XXXX (2026).
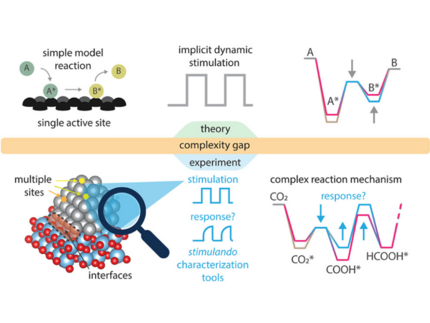
Traditional heterogeneous catalysis is constrained by kinetic and thermodynamic limits, such as the Sabatier principle and reaction equilibrium. Dynamic and resonant catalysts hold promise to overcome these limitations by actively oscillating a catalyst’s physical or electronic structure at the time scale of the catalytic cycle, allowing programmable control over reaction pathways, and leading to improved rate and selectivity. External stimuli such as temperature swing, mechanical strain, electric charge, and light can perturb catalyst surfaces in different ways, altering adsorbate coverage, binding energies, and transition states beyond what steady-state catalysis allows. This work surveys the current state of dynamic catalysis, introduces the concept of “stimulando” characterization for observing transient dynamics, and outlines key modeling, mechanistic, and benchmarking strategies to advance the field toward improved chemical transformation.
O. Verbitsky, S. Hinojosa, A. Mostafa, D. Ojha, I. Bald, and N. Kulak
Amphiphilic Cu(II) Oxacyclen Complexes: From Oxidative Cleavage to Condensation of DNA
ChemBioChem 2026, 27, e202500477
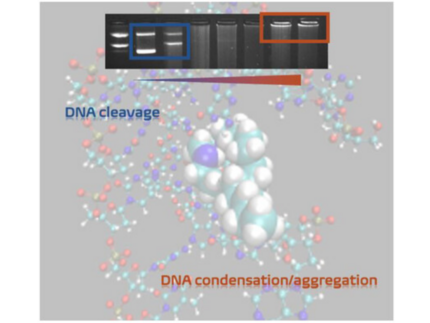
Cu(II) complexes with monoalkylated oxacyclen ligands (C12, C16, and C18) have been investigated regarding their interaction with DNA by different methods: circular dichroism, UV/VIS (ultraviolet-visible) and fluorescence spectroscopy as well as by gel electrophoresis. The results demonstrate that the complexes can cleave DNA through both hydrolytic and oxidative mechanisms, with hydroxyl radicals and hydrogen peroxide identified as the reactive oxygen species involved. The targeted incorporation of alkyl chains significantly enhances the DNA-binding affinity of the Cu(II) complexes, and the length of the alkyl substituents plays an important role, as they can interact with the major groove of the DNA. Alkylation is the determining structural factor responsible for the enhanced DNA interaction, since such an interaction is not observed with unsubstituted complexes. Moreover, the length of the alkyl chains significantly influences this behavior, as longer substituents induce a concentration-dependent DNA aggregation, a phenomenon absent in the nonalkylated analog. This aggregation and condensation behavior is examined using atomic force microscopy and dynamic light scattering. Moreover, DNA/small molecule interactions are also investigated using molecular dynamics simulations.
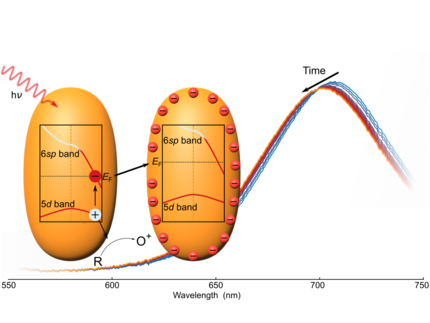
F. Stete, M. Bargheer, and W. Koopman
Capacitive photocharging of gold nanorods
Nature Communincations 17, 139 (2026)
Light can charge plasmonic nanoparticles by photoredox reactions, significantly modifying their optical and chemical properties. However, the charging process has been challenging to track experimentally, severely hindering its thorough evaluation. In this study, we investigate the charging of gold nanorods during a light-induced reaction in situ, utilizing the sensitivity of the rods’ longitudinal localized surface plasmon resonance to charge accumulation. Describing the particles as nanocapacitors, we present a model to quantify the number of charges on the particles and their connection to the illumination intensity. We find that the Fermi level, together with all other energy bands, is raised because of the repulsive potential of the additional charges. Experimental observations of the dependence on the solvent, the particle size, and ligand type further corroborate the proposed capacitor model. The results presented in this study lay the groundwork for the rational engineering of dynamic charge accumulation during plasmon-driven photoreactions.
Preprints
A. Zehle, C. Penschke, E. Titov.
Molecular Excitons in Arylazopyrazole Aggregates: A Quantum Chemical Study
Aggregation of molecular photoswitches may affect their functionality. Fundamentally, interaction of monomers in the aggregated state results in formation of exciton states, which, in turn, govern energy and charge transfer processes in the materials made of the photoswitches. In this work, we study the exciton states of aggregates of arylazopyrazole — the photoswitch which gained popularity in last decade as an alternative to azobenzene — using quantum chemical calculations. We perform cluster excited-state calculations for aggregates including up to 32 arylazopyrazole monomers as well as periodic calculations for the crystal structure. We obtain and analyze the composition of the exciton states, exciton splittings, and monomer-to-aggregate spectral shifts, thus providing quantitative insight into the electronic states and absorption spectra of realistic arylazopyrazole aggregates.
N. Pallab, M. Schenderlein, E. Titov, E. Sperlich, M. Schmette, and M. Reifarth
D-Fructose, L-Sorbose, D-Tagatose, D-Psicose: Functional Methacrylate-Based Glycopolymers of Ketohexoses Possessing Enhanced Boronic Acid Affinity
The unique binding properties of ketohexoses with boronic acids present new opportunities for functional material design. We investigated the binding of D-fructose, L-sorbose, D-tagatose, and D-psicose with four boronic acids (phenylboronic acid (PBA), 3-acetamidophenylboronic acid (AcPBA), benzoxaborole (BOB), and 6-acetamidobenzoxaborole (AcBOB)) in aqueous environment using isothermal titration calorimetry (ITC), finding that D-psicose exhibits exceptional affinity, compared to other ketohexoses. The ketohexose sugars were efficiently converted into isopropylidene-protected methacrylate monomers for polymer formation. These polymers can be deprotected in an acidic condition to yield the side-chain functionalized glycopolymers, providing a versatile platform for strong boronic acid–carbohydrate interactions for dynamic and advanced material design.