Research
Background
Vertebrate organs are derived from epithelial or endothelial sheets of cells that undergo complex morphogenetic transformations. In our lab, we study the embryonic zebrafish cardiovascular system which is relatively simple compared with that of mammals. We would like to better understand the signaling events that instruct the morphogenesis of the early heart tube. Initially the zebrafish heart consists of only the outer myocardial and inner endocardial cell layer, a specialized population of endothelial cells. We would like to understand: What are the signals that regulate the morphogenesis of myocardium and endocardium? To what extent do these two tissues communicate during cardiac looping and ballooning morphogenesis, and during the formation of cardiac valves? What determines the differentiation of endocardium into its different morphological derivatives such as valvular cells? In collaboration with clinical researchers, the group uses developmental genetics combined with cell biological and pharmacological approaches to develop animal models for human cardiovascular diseases of the heart and the vasculature.
We use highly interdisciplinary approaches to analyze cardiovascular morphogenesis by combining whole-genome expression analyses, functional cell biological and genetic tools for tissue- or single cell level functional studies, in vivo high-resolution 4D-confocal imaging, systems biological approaches, in silico modeling of single cell behaviors during cardiovascular morphogenesis and pathogenesis, and pharmacological studies.
Most important references during the past years
- Paolini A, Sharipova D, Lange T, Abdelilah-Seyfried S. Development. 2023 Sep 15;150(18):dev201707. doi: 10.1242/dev.201707. Wnt9 directs zebrafish heart tube assembly via a combination of canonical and non-canonical pathway signaling.
- Grdseloff N, Boulday G, Rödel CJ, Otten C, Vannier DR, Cardoso C, Faurobert E, Dogra D, Tournier-Lasserve E, Abdelilah-Seyfried S. Impaired retinoic acid signaling in cerebral cavernous malformations.
- Paolini A, Fontana F, Pham VC, Rödel CJ, Abdelilah-Seyfried S. Cell Rep. 2021 Oct 5;37(1):109782. doi: 10.1016/j.celrep.2021.109782. Mechanosensitive Notch-Dll4 and Klf2-Wnt9 signaling pathways intersect in guiding valvulogenesis in zebrafish.
- Fontana F, Haack T, Reichenbach M, Knaus P, Puceat M, Abdelilah-Seyfried S. (2020) Antagonistic Activities of Vegfr3/Flt4 and Notch1b Fine-tune Mechanosensitive Signaling during Zebrafish Cardiac Valvulogenesis. Cell Rep.;32(2):107883. doi: 10.1016/j.celrep.2020.107883. PMID: 32668254.
- Lombardo VA, Heise M, Moghtadaei M, Bornhorst D, Männer J, Abdelilah-Seyfried S.(2019) Morphogenetic control of zebrafish cardiac looping by Bmp signaling. Development. PubMed 146(22). pii: dev180091. doi: 10.1242/dev.180091.
- Bornhorst D, Xia P, Nakajima H, Dingare C, Herzog W, Lecaudey V, Mochizuki N, Heisenberg CP, Yelon D, Abdelilah-Seyfried S. (2019) Biomechanical signaling within the developing zebrafish heart attunes endocardial growth to myocardial chamber dimensions. Nat Commun. doi: 10.1038/s41467-019-12068-x.
- Rödel CJ, Otten C, Donat S, Lourenço M, Fischer D, Kuropka B, Paolini A, Freund C, Abdelilah-Seyfried S. (2019) Blood Flow Suppresses Vascular Anomalies in a Zebrafish Model of Cerebral Cavernous Malformations. Circ Res. doi: 10.1161
- Chapman EM, Lant B, Ohashi Y, Yu B, Schertzberg M, Go C, Dogra D, Koskimäki J, Girard R, Li Y, Fraser AG, Awad IA, Abdelilah-Seyfried S, Gingras AC, Derry WB. (2019) A conserved CCM complex promotes apoptosis non-autonomously by regulating zinc homeostasis. Nat Commun. doi: 10.1038/s41467-019-09829-z.
- Demal TJ, Heise M, Reiz B, Dogra D, Brænne I, Reichenspurner H, Männer J, Aherrahrou Z, Schunkert H, Erdmann J, Abdelilah-Seyfried S. (2019) A familial congenital heart disease with a possible multigenic origin involving a mutation in BMPR1A. Sci Rep. doi: 10.1038/s41598-019-39648-7.
- Otten C, Knox J, Boulday G, Eymery M, Haniszewski M, Neuenschwander M, Radetzki S, Vogt I, Hähn K,De Luca C, Cardoso C, Hamad S, Igual Gil C, Roy P, Albiges-Rizo C, Faurobert E, von Kries J P, Campillos M, Tournier-Lasserve E, Derry W B, Abdelilah-Seyfried S. (2018) Systematic pharmacological screens uncover novel pathways involved in cerebral cavernous malformations. EMBO Mol Med e9155 doi: 10.15252/emmm.201809155
- Merks A, Swinarski M, Meyer A, Müller N, Ozcan I, Donat S, Burger A, Gilbert S, Mosimann C, Abdelilah-Seyfried S, and Panakova D (2018) Planar Cell Polarity signalling coordinates heart tube remodelling through tissue-scale polarisation of actomyosin activity. Nature Communications. 9, 2161. doi: 10.1038/s41467-018-04566-1.
- Donat, S., Lourenço, M., Paolini, A., Otten, C., Renz, M., and Abdelilah-Seyfried, S. (2018). Heg1 and Ccm1/2 proteins control endocardial mechanosensitivity during zebrafish valvulogenesis. ELife 7, e28939 doi: 10.7554.
- Haack, T., Abdelilah-Seyfried, S. (2016) The force within: endocardial development, mechanotransduction and signalling during cardiac morphogenesis. Development 143, 373-386.doi: 10.1242/dev.131425
- Renz, M., Otten, C., Faurobert, E., Rudolph, F., Zhu, Y., Boulday, G., Duchene, J., Mickoleit, M., Dietrich, A.C., Ramspacher, C., Steed, E., Manet-Dupé, S., Benz, A., Hassel, D., Vermot, J., Huisken, J., Tournier-Lasserve, E., Felbor, U., Sure, U., Albiges-Rizo, C., Abdelilah-Seyfried, S. (2015) Regulation of β1 Integrin-Klf2-mediated angiogenesis by CCM proteins. Dev. Cell 32, 181-190doi: 10.1016/j.devcel.2014.12.016.
- Dietrich, A.C., Lombardo, V.A., Veerkamp, J., Priller, F., Abdelilah-Seyfried, S. (2014) Blood flow and Bmp signaling control endocardial chamber morphogenesis. Dev. Cell 30, 367-377doi.org/10.1016/j.devcel.2014.06.020
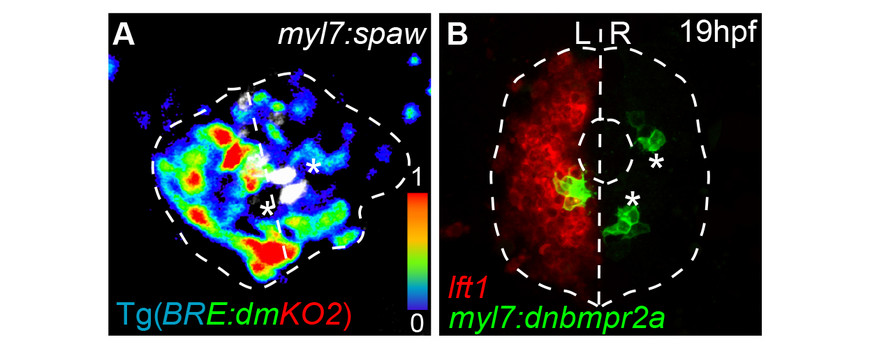
- Veerkamp, J., Rudolph, F., Cseresnyes, Z., Priller, F., Otten, C., Renz, M., Schaefer, L., Abdelilah-Seyfried, S. (2013) Unilateral dampening of BmP activity by nodal generates cardiac left-right asymmetry. Dev. Cell 24, 660-667doi: 10.1016/j.devcel.2013.01.026
- de Pater, E., Ciampricotti, M., Priller, F., Veerkamp, J., Strate, I., Smith, K., Lagendijk, A.K., Schilling, T.F., Herzog, W., Abdelilah-Seyfried, S., Hammerschmidt, M., Bakkers, J. (2012) BMP signaling exerts opposite effects on cardiac differentiation. Circ. Res. 110, 578-587 doi: 10.1161/CIRCRESAHA.111.261172
- Zhang, J., Piontek, J., Wolburg, H., Piehl, C., Liss, M., Otten, C., Christ, A., Willnow, T. E., Blasig, I. E., Abdelilah-Seyfried, S. (2010) Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion. Proc. Natl. Acad. Sci USA 107, 1425-1430doi: 10.1073/pnas.0911996107
- Lange, M., Kaynak, B., Forster, U.B., Tönjes, M., Fischer, J.J., Grimm, C., Schlesinger, J., Just, S., Dunkel, I., Krueger, T., Mebus S., Lehrach, H., Lurz, R., Gobom, J., Rottbauer, W., Abdelilah-Seyfried, S., Sperling, S. (2008) Regulation of muscle development by DPF3, a novel histone acetylation and methylation reader of the BAF chromatin remodeling complex. Genes Dev. 22, 2370-2384doi: 10.1101/gad.471408
- Rohr, S., Otten, C., Abdelilah-Seyfried, S. (2008) Asymmetric involution of the myocardial field drives heart tube formation in zebrafish. Circ. Res. 102, e12-19doi.org/10.1161/CIRCRESAHA.107.165241
- Cibrián-Uhalte, E., Langenbacher, A., Shu, X., Chen, J.N., Abdelilah-Seyfried, S. (2007) Involvement of zebrafish Na+, K+ ATPase in myocardial cell junction maintenenace. J. Cell Biol. 176, 223-230doi: 10.1083/jcb.200606116
- Rohr, S., Bit-Avragim, N., Abdelilah-Seyfried, S. (2006) Heart and soul/PRKCi and nagie oko/Mpp5 regulate myocardial coherence and remodeling during cardiac morphogenesis. Development 133, 107-115 doi: 10.1242/dev.02182