Amoeboid motility and actin dynamics
Actin dynamics in motile cells
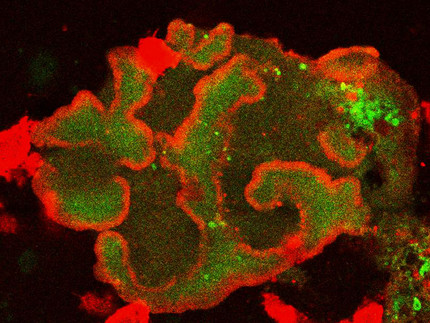
The dynamics of the actin cytoskeleton provides the basis for many essential cellular processes, including phagocytosis, cell division, and motility. These processes critically depend on the coordinated formation of functional cytoskeletal structures that exhibit characteristic length and time scales. The underlying spatiotemporal patterns in the actin cortex and the related signaling pathways are a major focus of our research. We study the emergence of these self-organized structures and develop techniques to manipulate them on a sub-cellular scale with the ultimate long-term goal to control and guide actin-driven cellular functions. Our experiments are performed with cells of the social amoeba Dictyostelium discoideum, a well-established model organism for actin dynamics and motility in eukaryotic cells.
- J. Negrete Jr., A. Pumir, H.-F. Hsu, C. Westendorf, M. Tarantola, C. Beta, and E. Bodenschatz, Phys. Rev. Lett. 117, 148102 (2016).
- M. Gerhardt, M. Walz, and C. Beta, J. Cell Sci. 127, 5115-5125 (2014).
- M. Gerhardt, M. Ecke, M. Walz, A. Stengl, C. Beta, and G. Gerisch, J. Cell Sci. 127, 4507-4517 (2014).
- C. Westendorf, J. Negrete Jr., A.J. Bae, R. Sandmann, E. Bodenschatz, and C. Beta, PNAS 110, 3853-3858 (2013).
- E. K. B. Pfannes, A. Anielski, M. Gerhardt, and C. Beta, Integr. Biol. 5 (12), 1456-1463 (2013).
Chemotaxis, Cell tracks and statistics
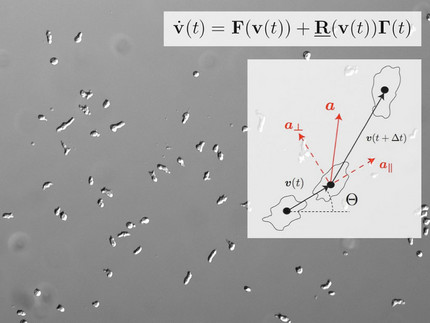
Among the actin-based cellular functions, amoeboid motility is one of the most prominent examples. It relies on the formation of membrane protrusions that are generated by coordinated localized events of actin polymerization. Extracellular chemical gradients can directionally guide this type of locomotion in a process called chemotaxis. A wide range of biological processes including wound healing, embryonic morphogenesis, and cancer metastasis, crucially depend on chemotactic cell motion. We have quantified the chemotactic response of Dictyostelium cells using microfluidic tools as a well-controlled and versatile experimental platform. Furthermore, we developed data-driven Langevin-type models to describe random and directed cell motion based on stochastic differential equations.
- G. Amselem, M. Theves, A. Bae, C. Beta, and E. Bodenschatz, Phys. Rev. Lett. 109, 108103 (2012).
- G. Amselem, M. Theves, A. Bae, E. Bodenschatz, and C. Beta, PLoS ONE 7(5), e37213 (2012).
- H.U. Bödeker, C. Beta, T.D. Frank, and E. Bodenschatz, EPL 90, 28005 (2010).
- L. Song, S.M. Nadkarni, H.U. Bödeker, C. Beta, A. Bae, C. Franck, W.-J. Rappel, W.F. Loomis, and E. Bodenschatz, Eur. J. Cell Biol. 85, 981-989 (2006).
Confinement and transport
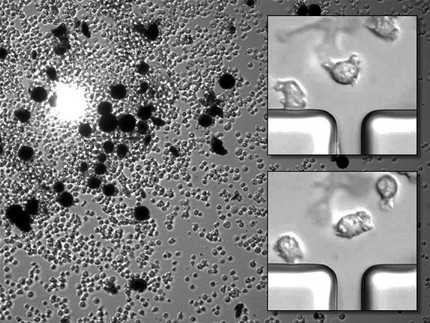
Our understanding of cell polarity and actin-driven motility mostly depends on studies of cells on planar open substrates. However, in their natural habitat, motile eukaryotic cells, such as leukocytes, cancer cells, and amoeba, typically move under very different conditions. They are confined inside the narrow interstitial spacings of tissue or soil and come into contact with other micron-sized objects that they may drag along. We use different micro-manipulation techniques to study the transport properties of amoeboid cells and how their polarity formation and motility is altered under such conditions.
- O. Nagel, C. Guven, M. Theves, M. Driscoll, W. Losert and C. Beta, PLoS ONE, 9 (12), e113382 (2014).
Adhesion dynamics
Our interest in cell adhesion concentrates on aspects related to cell locomotion. For efficient amoeboid motility, an optimal tuning of cell-substrate adhesion is essential. We use electric cell-substrate impedance sensing (ECIS) to study adhesion dynamics of motile cells. In particular, we focus on ECIS recordings of single motile Dictyostelium cells on microelectrodes and explore the potential of combining ECIS with microfluidics for applications in biotechnology.
- Helmar Leonhardt, Matthias Gerhardt, Nadine Höppner, Kirsten Krüger, Marco Tarantola, and Carsten Beta, Phys. Rev. E 93, 012414 (2016)
- E. Schäfer, C. Westendorf, E. Bodenschatz, C. Beta, B. Geil and A. Janshoff, Small 7, 723-726 (2011).